Autor : Luque, Graciela F.1, Melillo, Karina C.3, Lombardero, Lorena A.3, GonzÃĄlez, Norma E.4, Bisero, Elsa D.3 Diagnostic study group for TB GonzÃĄlez, Claudio D.1; SÃmboli, Norberto F.2; BiserÃģ, Elsa D.3; Luque, Graciela F.3; Melillo, Karina C.3; Lombardero, Lorena A3; GonzÃĄlez, Norma E.4; Amiano, NicolÃĄs O.5; GarcÃa, VerÃģnica E.5; DurÃĐ, Roberto M6; Armitano, Rita I.7; Fruhwald, Gladys E.8; Cerqueiro, MarÃa C.9
1Pulmonology and Tisiology Unit, Hospital General de Agudos JosÃĐ M. Ramos MejÃa, City of Buenos Aires. Argentina. 2Mycobacteria Service, Instituto Nacional de Enfermedades Infecciosas Dr. Carlos G. MalbrÃĄn, INEI-ANLIS. City of Buenos Aires, Argentina. 3Pediatric Service. Pediatric Pulmonology Section, Hospital Nacional Prof. Dr. Alejandro Posadas. El Palomar, Province of Buenos Aires. Argentina. 4Pulmonology and Tisiology Unit, Hospital General de NiÃąos Pedro de Elizalde. City of Buenos Aires. Argentina. 5Researchers from CONICET (National Scientific and Technical Research Council) Immunology and Tuberculosis Laboratory of the Institute of Biological Chemistry, Faculty of Exact Sciences and natural Sciences (IQUIBICEN), University of Buenos Aires (UBA), City of Buenos Aires. Argentina. 6Bronchoscopy Unit, Hospital de Infecciosas Francisco J. MuÃąiz. City of Buenos Aires. Argentina. 7Mycobacteria Laboratory. Hospital General de Agudos Parmenio PiÃąero. City of Buenos Aires. Argentina. 8Pulmonology Service of the Obra Social del Personal de Edificios de Renta y Horizontal (OSPERYH). 9Consultant of the Tisiology Section. Hospital de NiÃąos Dr. Ricardo GutiÃĐrrez. City of Buenos Aires. Argentina.
https://doi.org/10.56538/ramr.WNWY6973
Correspondencia : Claudio D. GonzÃĄlez. E-mail: claudiodgonzalez57@gmail.com
Received : 10/11/2022
Accepted : 11/01/2023
DIAGNOSIS OF TB IN PEDIATRIC PATIENTS
1. Approach to the diagnosis of childhood tuberculosis (CTB)
Dra. Elsa BiserÃģ
The incidence of childhood
tuberculosis (CTB) is often underestimated due to the difficulty of diagnosing
the disease in children. The clinical presentaÂtion of TB in pediatric patients
shows significant variability, with sometimes oligosymptomatic forms that occur
in a latent manner. Pediatricians must make an effort to understand this
disease and prevent its progression to severe forms, thus contributing to its
epidemiological control.
In 2020, the WHO (World Health
Organization) estimated that globally, each year, 1,100,000 children under 15
years old fall ill with TB, and among them, 226,000 lose their lives. This
represents approximately 11 % of the total number of TB cases. 80 % of these
deaths occur in children under 5 years of age, and 17 % in patients infected
with HIV (human immunodeficiency virus). It is estimated that between 25,000
and 32,000 children under 15 years old develop
multidrug-resistant tuberculosis (MDR-TB) each year. Only 12,220 of them
started treatment between 2018 and 2020.1, 2
In Argentina, during 2020, 10,896
cases of tuberculosis were reported, of which 10,268 were new cases and
relapses. 17 % of the cases correspond to children and adolescents. 76.9 % of
the newly diagnosed cases were pulmoÂnary.3
Childhood tuberculosis (CTB) can
be diagnosed relatively simply and, in the vast majority of cases, it can be
cured with low-cost, well-tolerated treatÂments. Nevertheless, it continues to
pose a chalÂlenge for public health.4
The purpose of this
chapter is to provide approÂpriate guidelines for the diagnostic methodology,
describe its characteristics (sensitivity and specificÂity performance), and
formulate recommendations for its use.
The goal is to establish a
set of recommendaÂtions for detecting suspected cases of CTB based on the best
available scientific evidence and expert consensus.
The mission of this
section is aimed at detecting children with TB, and the vision is
constructed in terms of patientsâ needs, system requirements, and the
individualsâ quality of life.
The overall objective of
this chapter is to provide updated tools for the diagnosis of CTB.
2. The role of the anamnesis in the diagnosis of CTB
Dr. Karina Melillo
The anamnesis is fundamental and
should be thorÂough and systematic. The first step in the diagnosis is to suspect
the possibility of TB. In any outpatient or hospital consultation, it is
imperative to gather personal and environmental information regarding
manifestations of chronic disease compatible with TB. When taking a detailed
anamnesis, important epidemiological aspects should be considered both for the
patient and also from the perspective of public health. This assessment
encompasses variÂous aspects.
As personal history,
considerations include BCG vaccination, the presence of a post-vacciÂnation
scar, and a prior tuberculin test (TT), including its date of administration
and result. If the individual received previous anti-tuberculosis treatment or
chemoprophylaxis, it is necessary to determine the date, drugs used, duration,
any intolerance, instances of discontinuation, and inÂterruptions.
Additionally, itâs important to identify comorbidities, immunodeficiencies, and
potential immunosuppressive treatment.
Regarding the patientâs family
history, it is important to inquire about any focus studies, the history of
current TB cases or those within the last two years in the patientâs
environment, cliniÂcal symptoms, and therapeutic measures taken. It is crucial
to specify the duration of exposure, whether it is a bacillary or cavitary
case, the drug resistance status, to record treatment adherence, and ensure a
comprehensive study of contacts. Furthermore, maintaining open communication
with the professionals overseeing the index case is essential.
If the child is the index case,
meaning that no known TB patient emerges during the anamneÂsis, it is essential
to search for the bacillary focus among contacts with respiratory symptoms
(RS), as it could be a patient who has not yet been diÂagnosed.5-7
In children, TB is considered a
sentinel event, indicating recent community transmission from a bacillary
adult.7, 8
All children at risk of having TB
should be studÂied and classified as exposed, infected, or sick. Each stage
requires a different therapeutic approach, and certain factors, such as a
history of present illness, should be carefully evaluated.8
The age at which the infection
occurs and the immune status are the two most
important variÂables determining progression to disease. Babies and toddlers,
especially those under 2 years old, have a high probability of developing
active disÂease, with the majority experiencing it within a year of the
primo-infection.9 Additionally,
these young children have a greater risk of developing severe and disseminated
forms of the disease, which are often fatal. The risk is lower for those
between 5 and 10 years old and increases again during adolescence. Itâs
important to note that most immunocompetent children infected with M.
tuberculosis will not become sick, but immuÂnodeficient individuals should
always be studied in areas where TB is prevalent.8
The systematic detection of TB
disease in children is a challenge. Diagnostic tools are less accurate in
children than in adults. Children have lower yield from microbiological tests.
Bacteriological confirmation in the pediatric population is achieved in less
than 40 % of the cases. Since the reference method (gold stanÂdard) for the
diagnosis is still the direct detection of M. tuberculosis in biological
samples or its cultivation in specific media, the delay in awaiting results can
lead to late diagnosis and treatment.
3. The role of clinical symptoms in the diagnosis of CTB
The clinical presentation of
TB is highly variable and can be less expressive (in the majorÂity of cases),
leading to diagnostic delays, disease progression, and new infections.
The onset is often insidious and
chronic. SympÂtoms vary with age, TB type (primary or post-priÂmary), the
extent of the disease, and the patientâs immune status.
The most common presentation of
TB in chilÂdren is the gangliopulmonary form, and most of these patients will
exhibit few symptoms or be asymptomatic.8
Signs and symptoms result from
the compresÂsion of the airway and parenchyma by enlarged lymph nodes; this is
more common in infants and young children due to the smaller caliber of the
airways. They often present with a dry cough (perÂsistent for more than two
weeks), mild dyspnea, and fixed, persistent, asymmetric wheezing that does not
respond to bronchodilator treatment, whether with or without tachypnea. In
children under 3 years old, symptoms may mimic viral infections, and this
creates diagnostic challenges. Weight loss or poor weight gain, along with
fever (with or without night sweats), occur with the progression of the
disease, reaching 89 % sensiÂtivity and 69 % specificity for detecting CTB.4 In children
under 3 years old, sensitivity drops to 50 %. In this age group, it is
important to screen for lethargy and decreased activity (decreased play), as
cough may be absent.10,
11 A
recent Cochrane review concludes that the combination of one or more symptoms
would have a sensitivity of 89 % in detecting pulmonary TB in children who are
in close contact with TB cases.11 The diagnosis
of TB should also be ruled out in children with severe pneumonia that does not
improve with the apÂpropriate antimicrobial treatment, or with pleural effusion
(more common in children under 2 years old and immunosuppressed), raising the
level of suspicion (see Table 1).2, 7, 8, 12-14
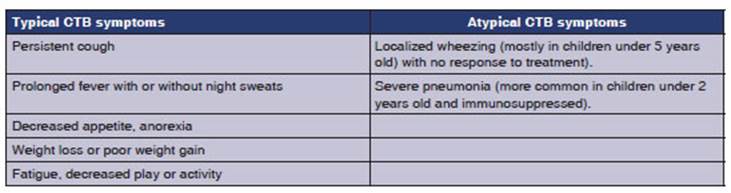
Towards the end of childhood and
during adolesÂcence (≥ 10 years of age), primary forms as those
described, or post-primary forms of reinfection (endogenous or exogenous) may
occur, similar to those found in adults. It is more likely that these patients
will experience classic symptoms of bacillary impregnation, such as evening
fever, anorexia, malaise, weight loss (documented in the last 3-6 months, or a
loss greater than 10 % of body weight in any time interval, unresponsive to
nutritional treatment), night sweats, persistent cough lasting more than two
weeks, either dry or productive with purulent or hemoptoic mucous exÂpectoration,
pleuritic chest pain, and hemoptysis. Physical examination findings are usually
milder than in younger children or may even be absent. Most of them present
with normal respiratory auscultation, even when there are cavities or large
infiltrates.10,
13
In extrapulmonary forms of TB,
the signs and symptoms corresponding to the affected organ, apparatus, or
system predominate.10
âĒ Non-painful adenopathy,
especially larger than 2 Ã 2 cm, with or without fistula. Nodal
TB.
âĒ Spinal kyphosis with narrow
angle (angular swelling), especially if it is of recent onset (gibÂbus). Spinal TB.
âĒ Signs of non-acute meningitis
(lasting for more than 5 days), especially if unresponsive to antiÂbiotic
treatment or with elevated intracranial pressure. Meningeal
TB.
âĒ Pleural effusion, especially
unilateral dullness with pleuritic pain in a child who is not acutely ill. Pleural TB.
âĒ Pericardial effusion, distant
or muffled heart sounds, or signs of new-onset heart failure. Pericardial TB.
TB infection. Table 2 shows the cutoff values used in our country
for interpreting results.3, 19

Epidemiological
research has indicated that the hostâs genetic component contributes to
infection and disease phenotypes, influencing both suscepÂtibility and
resistance. Approximately 30 %-50 % of exposed cohabiting contacts do not
become infected. Several family studies have provided consistent evidence of the
significant role of human genetics in the control of M. tuberculosis infection
or reactivity to PPD.20
Conversion
or tuberculin shift
Conversion or
tuberculin shift occurs when a tuberculin-negative individual becomes tubercuÂlin-positive,
or there is a difference of more than 10 mm between two readings within a
period of less than 2 years. This is considered indicative of recent infection.19
The negativity of the TT in chilÂdren does not exclude
the diagnosis of tuberculous infection or disease. Therefore, it should be
noted that it does not constitute a diagnostic element in itself but rather an
additional criterion to consider when evaluating a positive reaction to
tuberculin.
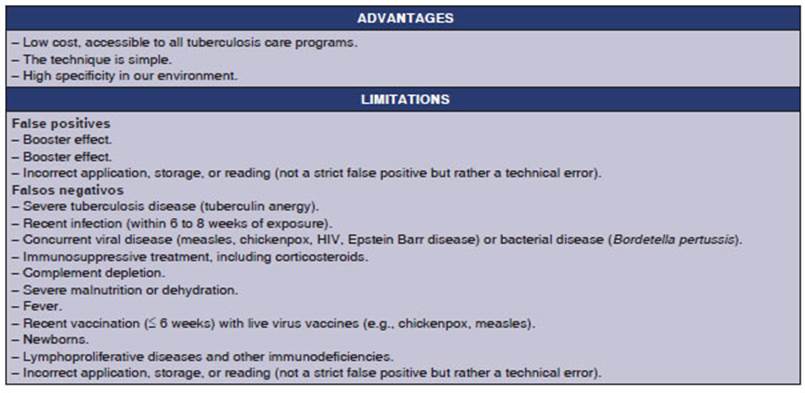
Inconveniences of
tuberculin Some of the protein elements of PPD (Purified Protein
DerivaÂtive) are shared by environmental mycobacteria and M. bovis (BCG),
and this reduces the specificity of the test. The lack of results for being
absent at the second reading visit worsened by the inability to repeat the test
immediately.21
Table 3 summarizes
the advantages and disadÂvantages provided by the TT.
Latest
tests in the diagnosis of the infection
The new tests for diagnosing TB
infection are based on concepts similar to PPD and IGRA, that is, eliciting an
immune response to specific antigens of M. tuberculosis, either in
vivo (skin induration size) or in vitro (magnitude of cytokine
release). There is still a pending need for a test that not only correctly
identifies healthy carriers but also those at a high risk of developing the
disease, in order to intervene with treatment.
In general, the
predictive performance of any new TB infection test should not be inferior to
curÂrent technology. IGRAs (QFT and T-SPOT) have become routine tests for
diagnosing presumed M. tuberculosis infection in low-prevalence
countries, either alone or in combination with PPD.
IGRAs are more specific
and require only one visit for phlebotomy. However, a second visit is often
necessary to clarify the result, rule out active TB, and start prevention.
IGRAs also have several disadvantages, including the need for complex
laboratory infrastructure and sample transportaÂtion, higher costs, variability
within individuals, and a high proportion of indeterminate results in advanced
HIV and very young children. They can yield false positives with
non-tuberculous mycobacteriosis, including M. marinum and M. kansasii.21,
22
Other tests: C-Tb (Statens Serum Institut Copenhagen, Denmark) and Diaskintest
(CJSC Generium, Russia) are skin reactions based on two specific antigens
of M. tuberculosis: ESAT-6 and CFP-10. They are applied and read in the
same way as the tuberculin test with PPD. They use a 5 mm induration as the
cutoff point regardless of the BCG or HIV status. Costs and sensitivity are
similar to the Mantoux test. They can be used for population testing and do not
cross-react with BCG.23, 24
Conclusion: in the context of diagnostic algoÂrithms for pediatric
TB, the tuberculin test and IGRAs are tools that provide specificity and allow
the identification of healthy carriers and those at high risk of developing
tuberculosis. They should not be interpreted in isolation.
6. The role of diagnostic imaging in CTB
6.1 The role of chest X-rays and computed
tomography scans
Dr. Norma GonzÃĄlez
The chest X-ray is a
valuable tool for the diagnoÂsis of CTB in pediatrics, as many children show
minimal symptoms at the onset of the disease, and pulmonary lesions are often
closed with a low quantity of bacilli (paucibacillary), making microbiological
confirmation challenging.
When interpreting
chest imaging, it is essential to recognize normal structures such as the
thymus or pulmonary vessels to avoid misclassifying an X-ray as pathological.
In this regard, inappropriÂate radiological techniques can also be confusing,
leading to mediastinal widening or increased pulmonary segments, in plates
taken during exÂpiration, rotated, or poorly penetrated. There is also
interobserver variability in the detection and interpretation of images,
particularly in cases of primary pulmonary TB in young children.25
Paratracheal,
intrathoracic, and perihilar adÂenomegalies are characteristic of primary TB.
To better identify them, it is preferable to evaluate both the frontal and
lateral views of the chest X-ray; the latter projection optimizes the visualÂization
of the lymph nodes in the mediastinum (Figure 1).
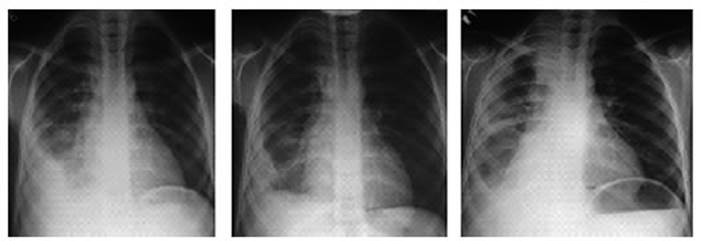
As the disease progresses,
intrathoracic lymph node lesions can compress the airways and cause atelectasis
(more frequent in the upper and right middle lobes) or hyperinflation.
The pulmonary
parenchymal involvement of TB manifests as pneumonia or bronchopneumonia not
radiologically distinguishable from that caused by other pathogens. However,
the persistence or worsening of these images despite appropriate anÂtibiotic
treatment raises suspicion of TB (Figure 2).
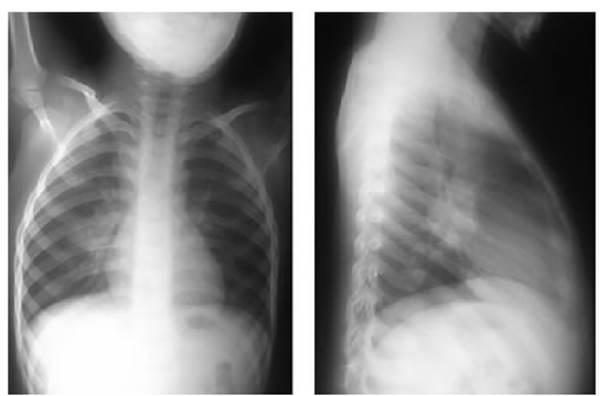
Single or multiple
cavitations, observed within opacities with diffuse borders usually located in
the upper lung fields, are highly suggestive of TB. They tend to occur in
children older than 10 years with respiratory symptoms, where bacteriological
confirmation is more common. Cavitations in lung lesions can also occur in
young children with a progressing disease.
Another radiological
pattern that prompts conÂsideration of TB is the disseminated micronodules in
both lung fields, characteristic of miliary TB (Figure 3).26, 27
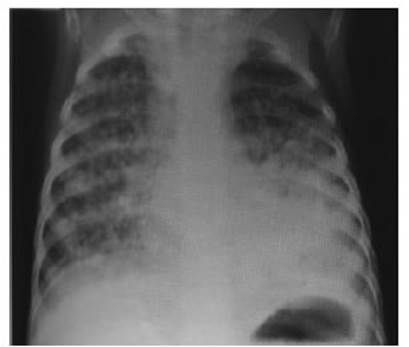
Many children have a
history of previous respiÂratory episodes or pre-existing conditions such as
asthma, post-infectious sequelae, HIV, or cystic fibrosis; in these cases,
X-rays should be compared with the patientâs previous imaging. When comparÂing
images, it is important to consider differential diagnoses of complications
that these conditions may present and look for images that raise suspiÂcion of
TB.
The ultrasound,
performed with appropriate techniques for pediatric patients by a trained
operator, can be useful for detecting adenomegaly and pleural effusion.28
Chest CT scans
increase diagnostic accuracy in cases where X-rays are inconclusive, especially
for detecting mediastinal adenomegalies. However, it is essential to consider
the increased radiation to which the patient is exposed and the need for
sedation or anesthesia to obtain good images in infants and young children.29
Currently, automated,
digital (computer-assistÂed), and tele-reading tools are being developed and
optimized for pediatric patients to simplify the interpretation of imaging
findings.28
6.2 The role of the ultrasound
Dr. Elsa BiserÃģ
Ultrasound is useful
for identifying and characterÂizing lesions in the pleura, chest wall,
diaphragm, and mediastinum. Its main advantages include the absence of ionizing
radiation, real-time exploration capability, the possibility of conducting the
study at the patientâs bedside, evaluating the extent of the
disease, and of performing sample collection, among others. These
characteristics are particuÂlarly useful in individuals more susceptible to the
adverse effects of radiation, such as children and pregnant women, or in
patients with difficulty in moving, such as those admitted to intensive care
units.30
In thoracic diseases, ultrasound
has played a secondary or practically nonexistent role. This is because 99 % of
ultrasounds emitted by the ulÂtrasound transducer are repelled at the interface
between the pleura and the lung. This is due to the significant difference in
acoustic impedance between soft tissues and air, as well as the substanÂtial
attenuation suffered by ultrasounds in their propagation through an air medium.31
Ultrasound in children is
indicated in the folÂlowing cases:
Study of serous
membranes
a. Study of the pleura. It
is more accurate (with 100 % sensitivity and 99.7 % specificity) than conÂventional
X-rays for detecting pleural effusions, as it can visualize as little as 5 mL
of fluid. The volume of the pleural effusion can be calculated usÂing various
equations based on the measurement of the lateral thickness of the fluid
column, the height of the subpulmonic effusion, and the thickness of the lung
covering. The simplest method involves multiplying the thickness of the lateral
fluid colÂumn (in mm) by an empirical factor of 90, resulting in the volume of
pleural effusion in milliliters (r = 0.68). It is a non-invasive,
cost-effective procedure, widely used in pleural TB. The ultrasound appearÂance
of a pleural effusion depends on its nature, cause, and chronicity. It allows
the detection of septa (thick or thin and mobile), the characterisÂtics of the
content (internal echoes), thickening, obtaining histological samples of
pleural lesions, with a success rate of 80 %, and dynamic monitorÂing of
lesions. Pleural drainage techniques can be applied under ultrasound guidance,
allowing the placement of smaller-caliber tubes with greater precision. Therefore,
ultrasound-guided thoracenÂtesis is a safe technique and can be performed in
patients on mechanical ventilation.31, 32
b. Study of the pericardium. In the diagnosis of pericardial effusion, color Doppler echocardiogÂraphy
is used to assess possible complications, such as cardiac tamponade or
constrictive pericarditis.33
c. Study of adenomegalies: Adenomegaly is defined as the presence of lymph nodes larger than 1 cm
in diameter. The increase in lymph node volume is due to various mechanisms:
1. Repeated antigenic stimulation
leading to lymÂphoid follicular hyperplasia with proliferation of intrinsic
cells in the node (lymphocytes or plasma cells).
2. Infiltration by cells external
to the node (LangÂerhans cell histiocytosis, deposition diseases).
3. Infiltration of
polymorphonuclear cells (infecÂtious adenitis).
4. Infiltration by tumor cells
(in leukemia and solid tumors).
Due to their evolution,
adenomegalies can be acute (less than 10 days of evolution), subacute (between
10 and 30 days), and chronic (more than 30 days of evolution). According to
their location, they can be localized or generalized.
Thoracic adenomegalies receive
lymph from the lung, heart, thymus, and esophagus. But in TB they can manifest
with symptoms such as cough, wheezing, dysphagia, hemoptysis, airway erosion,
atelectasis, neurological symptoms, and obstrucÂtion of large vessels (such as
the superior vena cava, with potential life-threatening implications for the
patient).
Ultrasound allows for the
specification of size, shape, echogenicity, vascularity, content (solid or
liquid), visualization of compressions, and access to certain areas of the
mediastinum, especially the anterior compartment and the aortopulmonary window.
It is used in cases with uncertain mediastinal images, (especially in children
under 2 years old) through suprasternal and parasternal approaches. It has been
demonstrated that up to 60 % of asymptomatic children with TB and chest X-rays
interpreted as normal may have mediastinal adenopathies, especially subcarinal.
The overall sensitivity of the ultrasound for the study of mediastinal
adenopathies is 62 %, rising to 72 % when conÂsidering accessible areas of the
lung. Guarda et al mention that adenopathies are present in 95 % of children,
but X-rays may not always show them.34-36
The work of Lisa C. Ruby et al
mentions that the proportion of children with lymphadenopathy detected by
mediastinal ultrasound ranged from 15 % to 85 %, and studies including
suprasternal and parasternal exploration achieved higher detecÂtion rates.
Three retrospective studies reported mediastinal lymphadenopathy on ultrasound
for the majority of cases that presented with a normal or inconclusive X-ray.36
c. Study of abdominal structures:
It allows visualizing ganglion structures, especially
the groups for aortic, cava, and mesenteric. In TB, lymph nodes can be discrete
or appear as opaque masses in clusters. It is common for enlarged nodes to
contain hypoechoic areas or calcifications in the late stages of TB.
Furthermore, the ultrasound alÂlows highlighting caseating granulomas,
especially in the liver and spleen, visualizing a primary nodal complex in the
hepatic hilum (the only pathogÂnomonic lesion of congenital TB). One drawback
is that intestinal gas can make it difficult to see different structures.34,
37
Endoscopic ultrasound (EUS) can
help obtain images of lesions near the gastrointestinal lumen, which can be
aspirated or biopsied using fine needle aspiration. Specific biopsies can be
taken from the lymph nodes, the liver, and the pancreas. The ultrasound is
useful for obtaining images of peritoneal tuberculosis.
6.3. Endobronchial ultrasound-guided transbronchial needle aspiration
(EBUS-TBNA)
Endobronchial ultrasound (EBUS)
is a minimally invasive technique that allows sampling of tissue from
peripheral lung lesions or mediastinal/hilar masses, with high diagnostic
precision and signifiÂcantly lower morbidity and mortality compared to
alternative approaches. In pediatric patients, EBUS-TBNA is primarily used to
diagnose meÂdiastinal adenopathies. This has led to increased diagnostic
success with a reduced rate of complicaÂtions. Due to the limited cooperation
of children and concerns from their parents, sampling lymph nodes can be
challenging. EBUS is a safe approach that demonstrates excellent sensitivity,
specificÂity, and precision, making it a valuable tool for pediatricians.37
A multicenter study
retrospectively analyzed 67 pediatric participants who underwent endobronÂchial
ultrasound-guided transbronchial needle asÂpiration (EBUS-TBNA) or endoscopic
ultrasound with bronchoscope-guided fine needle aspiration (EUS-B-FNA). With
the exception of two patients whose EBUS did not detect any significant lymph
node, an adequate sample was obtained in 60 parÂticipants (92.3 %) and a
diagnostic sample was obÂtained in 37 participants (56.9 %). The sensitivity of
EBUS-TBNA/EUS-B-FNA was 79.1 %, and the diagnosis was modified in 28
participants (41.8 %). The authors of this study considered EBUS-TBNA and
EUS-B-FNA to be safe and effective diagnostic methods for the evaluation of
children with mediÂastinal lymphadenopathy.38
Advantages: EBUS can clearly show the relaÂtionship between blood vessels, lymph
nodes, and lesions occupying space in the extraganglionar mediastinum.
Disadvantages: Although EBUS has many advantages, there are still some drawbacks. The
specific structure of the bronchi in children poses some obstacles. The smaller
size of the pediatric trachea and its deeper position limit the use of the
method. The incidence of device breakage is high, and the cost of repair is
high. Its application is contraindicated in patients with severely reduced lung
function or respiratory failure, excessively deteriorated cardiac function,
massive hemoptysis, and overall debilitating conditions. The diagnostic
accuracy depends on many factors such as needle size, operator experience, and
the location of lymph nodes.39, 40
7. Microbiological testing/clinical scope of application
Dr. Graciela Luque
There are several challenges in
the confirmation of the CTB diagnosis that arise from subtle or nonspecific
radiographic results and from the paucibacillary nature of the disease. A
confirmaÂtory diagnosis is based on the direct detection of the pathogen; alternative
approaches include detecting the immunoresponse or compatible histology. Direct
pathogen-based tests include culture, nucleic acid amplification tests (NAAT),
and bacilloscopies. To date, there isnât any accurate diagnostic test for CTB.
Therefore, it is essential for physicians to recognize that TB is often a cliniÂcal
diagnosis, since a negative test does not rule out the disease in children.
Samples for culture from symptomatic children and those with manifestations of
pulmonary or extrapulmonary lesions should always be studied.21, 41, 42
Selection, collection and
transportation of samples
It is essential to consider both
the training of personnel collecting samples with standardized protocols and
providing explanations to the paÂtient or their companion. The operatorâs
skill, the quality of the obtained sample, and transportation are requirements
that have an influence on the diagnosis.
Samples should be taken under
respiratory isolation conditions, safeguarding the operator, in sterile
containers, and should be transported to the laboratory in rigid, waterproof
containers with a tight seal. The accompanying documentation must be clear and
complete, including personal informaÂtion, the type of sample being sent,
conditions, diagnosis, and requested tests.43
Efforts have been made to enhance
the perforÂmance of the microbiological diagnosis using a wide variety of
sample types and, even combine different methods or use sample pooling.44,
45
Collecting a deep respiratory
sample for culture from a young child poses additional challenges. Most
children under 7 years old lack the cough strength or oral motor coordination
to produce a high-quality expectorated sputum sample; they need semi-invasive
techniques such as gastric aspiration/lavage or induced sputum (with or without
nasopharyngeal aspiration). Both methods have similar microbiological
performance, with a sensitivity between 30 % and 50 %.46,
47
An alternative method for
obtaining respiraÂtory samples involves using the string test, where a gelatin
capsule containing a nylon string (sweet string) is swallowed and later
retrieved for culture.48 Though promising,
children under four years old have shown swallowing difficulties with this
method, and it has been investigated in small groups with results similar to
those of induced sputum.49
Bronchoalveolar lavage (BAL) is
an invasive procedure requiring specialized training, not well-tolerated in
children. It is indicated for specific circumstances of suspicion with other
negative samples, in the differential diagnosis, or when there is no response
to treatment. Its performance depends on the type of lesion being studied and
the classical or molecular isolation method. 50
Nasopharyngeal aspiration (NPA)
and oral swab are still potential alternative samples. Further evidence is
needed before it can be recommended as a sample collection method for children.46
Fecal samples (stool tests) with
buffers have low sensitivity in children (32 % to 68 %). They are primarily
used in HIV-positive patients for molecular techniques.51
In extrapulmonary TB, the
performance varies, and samples must be representative of the infecÂtion site,
they shall be collected aseptically, and rapidly stored and transported to the
laboratory to minimize multiplication of contaminating organs. Ideally, samples
should reach the laboratory on the day of the collection. If transportation to
the laboratory is delayed by more than an hour, the samples must be
refrigerated at 4°C, both during transportation and upon arrival at the
laboratory, until they are processed.52
Cerebrospinal fluid (CSF) should
be processed immediately or refrigerated for less than 12 hours. In the case of
urine, two samples are obtained on consecutive days or every other day, and the
largÂest possible volume is submitted (including the collection of several
urine samples). The sample should be processed immediately because the acidic
pH affects the viability of the bacillus. For other fluids (pleural, ascitic, synovial),
the performance is not significantly higher than that from respiraÂtory
samples, but it improves when combined with biopsies or other
non-microbiological methods.53
In peripheral lymph node TB or
abscesses, mateÂrial is obtained through aspiration, fine needle asÂpiration,
or biopsy. Cotton swabs or balls shouldnât be used. For tissue samples or
biopsies, desiccation should be avoided by adding sterile distilled water.
In disseminated forms and in
immunocomÂpromised patients, blood cultures should also be performed.52
8. Histopathology
Dr. Karina Melillo
The histopathological diagnosis
is most frequently made in extrapulmonary TB.54 The overall perforÂmance
is not well characterized and depends to some extent on the experience of the
operator and pathologist; the sensitivity and specificity may be hindered by
other granulomatous processes.
The biopsy and autopsy allow us
to determine the presence of acid-alcoholic resistant bacilli (AARB) through
the histopathological study and confirmatory special studies, such as the
Ziehl- Neelsen (ZN) technique.54
The Kinyoun stain is similar to
the ZN stain but does not use heat to enhance the uptake. FluoÂrochrome
techniques with auramine-rhodamine are based on the same basic principle but
allow for a faster and more convenient visualization of mycobacteria.
When examining sections stained
with hemaÂtoxylin-eosin (H&E), granulomatous aggregates are often
encountered. These aggregates consist of abundant histiocytes loaded with
bacilli of apÂproximately 3 μm or whirlwind-like
granulomas with Langhans-type multinucleated giant cells having their nuclei
arranged in a âCâ shape, with central necrosis, not always accompanied by
reactive lymphocytes (necrotizing granuloma). The presence of multiple
granulomas in different evolutionary stages, with central caseous necrosis,
suggests the diagnosis of TB, although multiple conditions can produce
granulomas.54 Exudative lesions (fibrinous exudates with alveolar invasion of
neutrophils) are more common in immunocomÂpromised individuals and have a worse
prognosis. Both types of lesions may coexist, depending on the course of the
disease.
DNA probe hybridization allows
for the rapid identification of the species isolated in culture and even from
paraffin-embedded tissue blocks.
Conflict of interest
The authors of this work have no
conflicts of interest to declare.
REFERENCES
1. World Health Organization. Rapid communication on updated guidance on the management of
tuberculosis in children and adolescents. Geneva: World Health Organization;
2021. https://www.who.int/publications/i/item/9789240033450
2. WHO consolidated guidelines on
tuberculosis. Module 5: management of tuberculosis in
children and adolescents. Geneva: World Health Organization; 2022. Licence: CC
BY-NC-SA 3.0 IGO.
3.
BoletÃn N.° 5 Tuberculosis y lepra en la Argentina AÃąo V - CoordinaciÃģn de
tuberculosis y lepra. DirecciÃģn de respuesta al VIH, ITS, hepatitis virales y
tuberculosis, Ministerio de Salud, Argentina. Marzo de 2022.
4. WHO operational handbook on
tuberculosis. Module 2: screening - systematic screening for tuberculosis
disease. Geneva: World Health Organization; 2021. License: CC BY-NC-SA 3.0 IGO.
https://www.who.int/publications/i/item/9789240022676.
5.
GutiÃĐrrez D, VÃĄsquez A. La tuberculosis infantil: Enfoque epidemiolÃģgico y
nuevas alternativas de diagnÃģstico. Rev Cs Farm y Bioq (PerÚ) 2014;2:93-100. Disponible en: http://www.scielo.org.bo/scielo.php?script=sci_arttext&pid=S2310-02652014000100011&lng=es.
ISSN 2310-0265
6.
Holcberg M, Zabala C, GutiÃĐrrez S, Sisto G, Sosa M, Giachetto G. Prevalencia y
caracterÃsticas de niÃąos con tuberculosis diagnosticados a partir de un caso
Ãndice. Uruguay 2012-2014. Arch Pediatr Urug 2016;87:S3-S10.
Disponible en: http://www.scielo.edu.uy/scielo.php?script=sci_arttext&pid=S1688-12492016000500001&lng=es
7.
PÃĐrez-VÃĐlez CM, Marais BJ. Tuberculosis in children. N Engl J Med 2012;367:348-61.
https://doi.org/10.1056/NEJMra1008049.
8. MartÃnez L, Cords O, Horsburgh
CR, Andrews JR. Pediatric TB Contact Studies Consortium. The risk of
tuberculosis in children after close exposure: a systematic review and
individual-participant meta-analysis. Lancet 2020; 395:973- 84.
https://doi.org/10.1016/S0140-6736(20)30166-5.
9.
Roya-Pabon CL, PÃĐrez-VÃĐlez CM. Tuberculosis
exposure, infection and disease in children: a systematic diagnostic approach.
Pneumonia 2016;8:23. https://doi.org/10.1186/s41479-016-0023-9.
10. Marais BJ, Gie
RP, Hesseling AC, et al. A
refined symptom-based approach to diagnose pulmonary tuberculosis in chilÂdren.
Pediatrics. 2006;118: e1350-9.
https://doi.org/10.1542/peds.2006-0519.
11. Vonasek B, Ness T, Takwoingi,
et al. Screening tests for active pulmonary
tuberculosis in children. Cochrane Database Syst Rev 2021;6::CD013693.
https://doi.org/10.1002/14651858.CD013693.pub2.
12.
Cruz Anleu ID, VelÃĄsquez Serratos JR. Tuberculosis infantil. ÂŋCÃģmo
diagnosticarla? [Childhood tuberculosis. How to diagnose it?]. Arch Argent Pediatr. 2012;110:144-51.
https://doi.org/10.5546/aap.2012.144.
13.
Ramos Amador JT, Berzosa SÃĄnchez A., Callejas Caballero I.,IllÃĄn Ramos M.. Tuberculosis pulmonar en PediatrÃa. Pediatr Integral 2021;15:76-90.
14. Marais BJ, Pai M. Recent advances
in the diagnosis of childhood tuberculosis. Arch Dis Child 2007;92:446-52. https://doi.org/10.1136/adc.2006.104976.
15. Migliori GB, Ong C, Petrone
L, D'Ambrosio L, Centis R, Gotelli D. The definition of tuberculosis infection
based on the spectrum of tuberculosis disease. Breathe 2021;17
210079.https://doi.org/10.1183/20734735.0079 2021.
16. Vieira JL, Foschiera L,
Ferreira ICS, Chakr VC. Performance of the quantification of
adenosine deaminase and determination of the lactate dehydrogenase/adenosÂine
deaminase ratio for the diagnosis of pleural tuberculosis in children and
adolescents. J Bras Pneumol. 2021;47:e20200558. https://doi.org/10.36416/1806-3756/e20200558.
17. Zhang M, Li D, Hu ZD, Huang
YL. The diagnostic utility of pleural markers for
tuberculosis pleural effusion. Ann Transl Med 2020;8:607-18.
https://doi.org/10.21037/atm.2019.09.110.
18.
Sociedad Argentina de PediatrÃa. ComitÃĐ Nacional de NeumonologÃa. Coordinadora:
Dra. Norma GonzÃĄlez. Criterios de diagnÃģstico y tratamiento de la tuberculosis
infantil. Consenso. Arch Argent Pediatr 2016;114:189. https://doi.org/10.5546/aap.2016.189.
19. Cobat A, Poirier C, Hoal E,
et al. Tuberculin Skin Test Negativity Is Under Tight Genetic Control of
Chromosomal Region 11p14-15 in Settings With Different Tuberculosis
Endemicities. J Infect Dis 2015;211:317-21. https://doi.org/10.1093/infdis/jiu446
20. Lewinsohn DM, Leonard MK, Lo
Bue PA, et al. Official American Thoracic Society/Infectious Diseases Society
of America/Centers for Disease Control and Prevention CliniÂcal Practice
Guidelines: Diagnosis of Tuberculosis in Adults and Children. Clin Infect Dis.
2017;64:e1-e33. https://doi.org/10.1093/cid/ciw694.
21. Chiappini E, Storelli F,
Tersigni C, Venturini E, Martino M, Galli L. QuantiFERON-TB Gold In-Tube test
performance in a large pediatric population investigated for suspected
tuberculosis infection. Paediatr Respir Rev 201932:36-47.
https://doi.org/10.1016/j.prrv.2019.03.010.
22. Ruhwald M, Aggerbeck H,
Gallardo RV, et al. Safety and efficacy of the C-Tb skin test to diagnose
Mycobacterium tuberculosis infection, compared with an interferon γ release assay and the tuberculin skin test: a phase 3, double-blind,
randomized, controlled trial. Lancet Respir Med 2017;5:259-68.
https://doi.org/10.1016/S2213-2600(16)30436-2.
23. Slogotskaya L, Bogorodskaya
E, Ivanova D, Sevostyanova T. Comparative sensitivity of the test with
tuberculosis recombinant allergen, containing ESAT6-CFP10 protein, and Mantoux
test with 2 TU PPD-L in newly diagnosed tuberculosis children and adolescents
in Moscow. PLoS ONE 2018. 13: e0208705. https://doi.org/10.1371/journal.pone.0208705
24. Starshinova A, Dovgalyk I,
Malkova A et al. Recombinant tuberculosis allergen
(DiaskintestÂŪ) in tuberculosis diagÂnostic in Russia (MetaAnalysis). Int J
Mycobacteriol 2020; 9:335-46. https://doi.org/10.4103/ijmy.ijmy_131_20.
25. Zellweger JP, Heinzer R,
Touray M, Vidondo B, Altpeter E. Intra-observer and overall agreement in the
radioÂlogical assessment of tuberculosis. Int J Tuberc Lung Dis. 2006;10:1123-6.
26. ConcepciÃģn ND, Laya BF,
Andronikou S, et al. Standardized radiographic interpretation of thoracic
tuberculosis in children. Pediatr Radiol. 2017;47:1237-48.
https://doi.org/10.1007/s00247-017-3868-z.
27. Jain SK, Andronikou S,
Goussard P, et al. Advanced imagÂing tools for childhood tuberculosis:
potential applications and research needs. Lancet Infect Dis. 2020;20:
e289-e297. https://doi.org/10.1016/S1473-3099(20)30177-8.
28.
Buonsenso D, Pata D, Visconti E, et al. Chest CT Scan for the Diagnosis of Pediatric
Pulmonary TB: Radiological Findings and Its Diagnostic Significance. Front. Pediatr. 2021;
9:583197. https://doi.org/10.3389/fped.2021.583197.
29.
De La Quintana Gordon FB, Nacarino Alcorta B. EcografÃa pulmonar bÃĄsica. Parte
1. EcografÃa pulmonar normal y patologÃa de la pared torÃĄcica y la pleura. Rev
Esp Anestesiol Reanim 2015;62: 322-36.
https://doi.org/10.1016/j.redar.2015.02.003
30.
Vollmer I, Gayete A. EcografÃa torÃĄcica. Arch Bronconeumol. 2010;46:27-34. https://doi.org/10.1016/j.arbres.2008.12.004.
31.
Oyonarte WM. Enfoque diagnÃģstico en el paciente con derrame pleural. Rev Med
Clin Condes - 2015;26:313-24.
https://doi.org/10.1016/j.rmclc.2015.06.008
32.
RamÃrez-Lapausa M., MenÃĐndez-SaldaÃąa A., Noguerado- Asensio A. Tuberculosis
extrapulmonar, una revisiÃģn. Rev Esp Sanid Penit 2015;17:3-11.
https://doi.org/10.4321/S1575-06202015000100002.
33.
Bilbao Sustacha JA, Peix Sambola MA, Alonso MartÃn DE, DÃaz LÃĄzaro J.
AplicaciÃģn de la ecografÃa clÃnica pediÃĄtrica en AtenciÃģn Primaria. En: AEPap
(ed.). Congreso de ActualizaciÃģn PediatrÃa 2019. Madrid: LÚa Ediciones 3.0;
2019. p. 495-506.
34.
Tovar DÃaz M, Tang VelÃĄsquez AM, Concha Mendoza ND. Tuberculosis extrapulmonar
en pediatrÃa: un reto diagÂnÃģstico. Rev. Medicas UIS 2013;26:45-58.
Disponible en: https://revistas.uis.edu.co/index.php/revistamedicasuis/article/view/3582.
35.
Guarda ME, Kreft J. Tuberculosis en el niÃąo ÂŋCÃģmo se diagnostica? Rev Med Clin Condes - 2017;28:104-10. https://doi.org/10.1016/j.rmclc.2017.02.011.
36. Ruby LC, Heuvelings CC,
Grobusch MP, Andronikou S, BÃĐlard S. Transthoracic mediastinal ultrasound in
childhood tuberculosis: A review. Paediatr Respir Rev. 2022;41:40-8.
https://doi.org/10.1016/j.prrv.2020.11.002.
37. Steinfort DP, Wurzel D,
Irving LB, Ranganathan SC. Endobronchial ultrasound in pediatric pulmonology.
Pediatr Pulmonol. 2009;44:303-8. https://doi.org/10.1002/ppul.20991.
38. Dhooria S, Madan K,
Pattabhiraman V, et al. A multicenter study on the utility
and safety of EBUS-TBNA and EUS-B-FNA in children. Pediatr Pulmonol.
2016;51:1031-9. https://doi.org/10.1002/ppul.23415.
39. Park M, Owles H, Williams A,
Williams B, Whittaker E, Kon OM. Pediatric Endobronchial
Ultrasound-Transbronchial Needle Aspiration Under Conscious Sedation for
Suspected Tuberculosis in London. Pediatr Infect Dis J. 2020;39:e329-
31. https://doi.org/10.1097/INF.0000000000002819
40. Gulla KM, Gunathilaka G, Jat
KR et al. Utility and safety of endobronchial ultrasound-guided transbronchial
needle aspiration and endoscopic ultrasound with an echobronchoscope-guided
fine needle aspiration in children with mediastinal pathology. Pediatr
Pulmonol. 2019;54:881-5. https://doi.org/10.1002/ppul.24313.
41. Cuevas LE, Petrucci R,
Swaminathan S. Tuberculosis diagnostics for children in high-burden countries:
what is available and what is needed. Paediatr Int Child
Health. 2012;32(Suppl 2):S30-7. https://doi.org/10.1179/2046904712Z.00000000076.
42.
Ministerio de Salud. Gobierno de El Salvador. GuÃa clÃnica para la atenciÃģn
pediÃĄtrica de la tuberculosis y la coinfecÂciÃģn TB-VIH. San Salvador, El
Salvador 2021. http://asp.salud.gob.sv/regulacion/default.asp
43. Starke J, Cruz AI. Diagnosing Childhood Tuberculosis: A Small Step Forward.
JAMA Pediatrics 2021;175: e206078.
https://doi.org/10.1001/jamapediatrics.2020.6078
44. Datta S, Shah L, Gilman RH,
Evans CA. Comparison of sputum collection methods for tuberculosis diagnosis: a
systematic review and pairwise and network meta-analysis. Lancet Glob Health
2017;5:e760â71. https://doi.org/10.1016/S2214-109X(17)30201-2.
45. Ioos V, Cordel H, Bonnet M.
Alternative sputum collection methods for diagnosis of childhood intrathoracic
tuberculosis: a systematic literature review. Arch Dis Child 2019;104:629-35. https://doi.org/10.1136/archdischild-2018-315453.
46. Ruiz JimÃĐnez M, GuillÃĐn
MartÃn S, Prieto Tato LM, et al. Induced sputum versus gastric lavage for the
diagnosis of pulmonary tuberculosis in children. BMC Infect Dis. 2013;
13:222-8. https://doi.org/10.1186/1471-2334-13-222.
47. Tafur KT, Coit J, Leon SR, et
al. Feasibility of the string test for tuberculosis diagnosis in children
between 4 and 14 years old. BMC Infect Dis. 2018;18:574-81.
https://doi.org/10.1186/s12879-018-3483-0.
48. Imperiale BR, Nieves C,
Mancino B, et al. String test: A new tool for tuberculosis diagnosis and
drug-resistance detection in children. Int J Mycobacteriol. 2018;7:162-6. https://doi.org/10.4103/ijmy.ijmy_54_18.
49. Goussard P, Retief F, Burke
J, Malherbe A, Janson J. The role of bronchoscopy in the
diagnosis and management of pediatric pulmonary tuberculosis. Ther Adv
Infectious Dis 2021;8:1-19. https://doi.org/10.1177/20499361211037168
50. Mesman AW, RodrÃguez C, Ager
E, Coit J, Trevisi L, Franke MF. Diagnostic accuracy of molecular detection of
Mycobacterium tuberculosis in pediatric stool samples: A systematic review and
meta-analysis. Tuberculosis (Edinb). 2019;119:101878. https://doi.org/10.1016/j.tube.2019.101878
51. Dunn JJ, Starke JR, Revell
PA. Laboratory diagnosis of MyÂcobacterium tuberculosis
infection and disease in children. J Clin Microbiol 2016; 54:1434-41. https://doi.org/10.1128/JCM.03043-15.10.1016/j.jctube.2020.100164
52.
Foppiano Palacios C, Saleeb PG. Challenges in the diÂagnosis of tuberculous meningitis. J Clin Tuberc Other Mycobact Dis 2020;20:100164.
https://doi.org/10.1016/j.jctube.2020.100164.
53. Bagdia M, Bijwe S, Hirani N,
Joshi A, Chowdhary A, Agrawal M, Bagdia A. Lab Diagnosis of Extra Pulmonary
Tuberculosis: Comparison of Histopathology, Cytology, ZeihlNeelsen stain and
Light Emission Diode Microscopy with Culture and Nucleic Acid Amplification
Tests. Int J Cur Res Rev 2018; 10:15-9.
https://doi.org/10.7324/IJCRR.2018.10803.
54. Shah KK, Pritt
BS, Alexander MP. Histopathologic
review of granulomatous inflammation. J Clin Tuberculosis Other
Mycobacterial Dis 2017; 7:1-12. https://doi.org/10.1016/j.jctube.2017.02.001