Autor : Moreno, Pablo1, Luhning, Susana2,YÃĄÃąez, AnahÃ1, Stock, Ana2, Gattolin, Gabriel1, Mattarucco, Walter2, Maillo, Martino1, HernÃĄndez, Marcos2, SÃvori, MartÃn2
1 Argentinian Association of Allergy and Clinical Immunology (AAAeIC) 2 Argentinian Association of Respiratory Medicine (AAMR)
https://doi.org/10.56538/ramr.KYEH5462
Correspondencia : Pablo Moreno. E-mail: secreÂtaria@aaaeic.org.ar
ABSTRACT
Asthma is a common chronic airway
disease in our country, although with high poor conÂtrol. Some specialists of
the AsociaciÃģn de Alergia e
InmunologÃa ClÃnica and AsociaciÃģn Argentina de Medicina Respiratoria have made recommendations for management and
treatment of asthma, using a RAND/UCLA modified Delphi consensus methodology,
based on GRADE evidence.
This document provides
recommendations based on specialist opinions about different strategies to
improve adherence. Besides, it provides recommendations about critical issues
of mild to severe asthma treatment.
ItÂīs recommended to improve
adherence, personalized control-based management plan (1 °C), mobile devices
(1B) and education (1 °C). Sublingual immunotherapy must be prescribed only in
patients with allergic rhinitis, mite associated, and persistent symptoms
although appropriate treatment with FEV1>
70 % (1B). Use of fast action bronchodilators associated with inhaled
corticosteroids prn in mild asthma (GINA stage 2) has
strong recommendation (1A). Use of triple inhaled therapy (long acting anticholinergics, long acting beta 2 agonists and inhaled
corticosteroids) is recommended in severe asthma (1B). Biologics has strong
recommendations severe asthma: in phenotype T2 with duÂpilumab
(1A), in phenotype allergic T2 with omalizumab (1A)
and phenotype eosinophilic T2 with benralizumab or mepolizumab with
distinctive characteristic (1A).
Key word: Asthma, Adherence, Immunotherapy, Triple therapy, Biologics
RESUMEN
El
asma es una enfermedad crÃģnica de la vÃa aÃĐrea prevalente en nuestro paÃs, con
frecuente mal control. Algunos especialistas de la AsociaciÃģn de Alergia e
InmunologÃa ClÃnica y la AsociaciÃģn Argentina de Medicina Respiratoria han
realizado recomendaÂciones sobre el manejo y tratamiento del asma mediante la
metodologÃa de consenso RAND/UCLA Delphi modificada sobre la base de la
evidencia cientÃfica (GRADE).
Este
documento provee recomendaciones basadas en la opiniÃģn de especialistas y
fundamentada en evidencia cientÃfica seleccionada en cuanto a la importancia de
mejorar la adherencia al tratamiento y seguimiento a travÃĐs de diferentes
estrategias. Asà mismo, provee recomendaciones actualizadas en aspectos
crÃticos del tratamiento del asma leve al grave.
Se
recomienda, para mejorar la adherencia, el uso de planes personalizados de
manejo (1 °C), uso de herramientas a travÃĐs de telÃĐfonos mÃģviles (1B) y
educaciÃģn (1 °C). Con respecto a la inmunoterapia sublingual solo debe ser
indicada a pacientes con asociaÂciÃģn con rinitis alÃĐrgica, asociada a ÃĄcaros y
sÃntomas de asma a pesar del tratamiento adecuado con FEV1 > 70 % (1B). Se recomienda fuertemente en
el asma leve (escalÃģn 2 GINA) el uso de broncodilatadores de acciÃģn rÃĄpida
asociados a corticoides inhalados a demanda (1A). En asma grave, se recomienda
el uso de la triple terapia inhalada con anticolinÃĐrgicos de acciÃģn prolongada,
beta 2 de acciÃģn prolongada y corticoides inhaÂladas (1B). El uso de biolÃģgicos
en asma grave estÃĄ fuertemente indicado en fenotipo T2 con dupilumab
(1A), T2 alÃĐrgico con omalizumab (1A) y en el T2 eosinofÃlico con benralizumab, o mepolizumab, con sus caracterÃsticas distintivas (1A).
Palabras
clave: Asma,
Adherencia, Inmunoterapia, Triple terapia, BiolÃģgicos
Recived: 08/07/2023
Accepted: 10/21/2023
INTRODUCTION
Asthma is a heterogeneous,
inflammatory airway disease characterized by recurrent episodes of
bronchospasm, bronchial hyperreactivity, and
increased bronchial secretions.1-3 It affects apÂproximately
300 million people around the world; in Latin America, there is great
heterogeneity reÂgarding its prevalence depending on each country, ranging from
5 % to 24 %, as is the case in Costa Rica.4
In Argentina, it is estimated that between 6.4 % and 9.36 % of
the population has asthma, according to different studies.5-6
The knowledge of the disease has
made signifiÂcant advancements in recent years, especially in terms of
diagnosis and treatment. Paradoxically, even though morbidity and mortality and
hospiÂtalizations have been reduced through preventive anti-inflammatory
treatment, there are still epideÂmiological indicators of poor control, and in
some countries, there are still surprisingly high percentÂages of
hospitalizations and mortality.1-3,
5, 7, 8
This new knowledge also generates
topics of discussion and points of interest that require local perspectives.
There are already several recent inÂternational guidelines and one local
guideline from a few years ago that cover the diagnosis and treatÂment of
asthma broadly. The objective is to address key questions with the highest
level of evidence, focusing on topics related to asthma management, in order to
create a practical and easily readable tool with recommendations that provide
original contributions aimed at the interests of physicians dedicated to
asthma.1-3, 9-10
This document was prepared by a
panel of specialists from the Association of Allergy and Clinical Immunology (AAAeIC) and the ArgentinÂian Association of Respiratory
Medicine (AAMR), with special emphasis on establishing scientific
evidence-based recommendations for the diagnosis and treatment of asthma in
adults, adapted to the local context.
MATERIALS AND METHODS
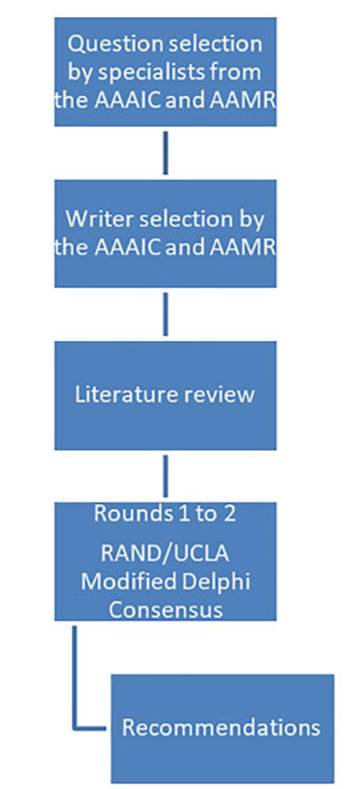
The convergence of both societies
allowed for the analysis of common areas. Representatives with extensive
experience in asthma management were selected from each society to choose
questions that addressed common discussion points and were clinically relevant
in our country regarding the diagnosis and treatment of asthma in adults.
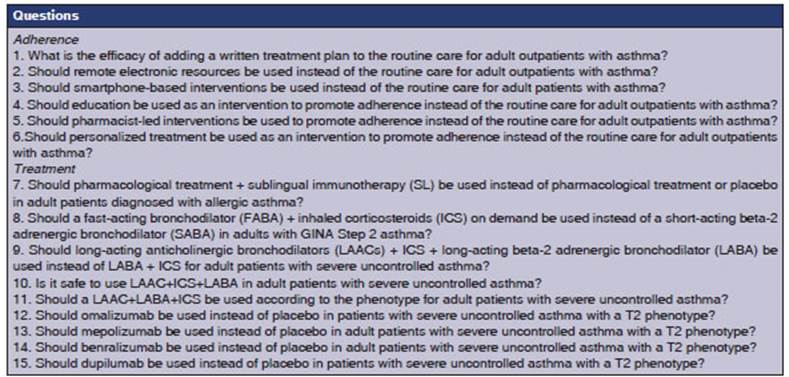
Twenty-eight specialists proposed twenty-two questions, assigning a score based
on both conditions. These questions were then ranked from highest to lowest
score, and the top 15 were analyzed (Table 1). For the purpose of preparing the
manuscript, the questions were grouped based on the topics they covered (seven
questions on adherence and eight on treatment), so that each question could be
discussed and analyzed and the corresponding recommendations could be
established (Figure 1).
Literature searches were conducted
in the MEDLINE, EMBAÂSE, Cochrane, SciELO, and Lilacs
databases until October 31, 2022, using search terms relevant to the respective
questions. WE used the GRADE system (Grading of Recommendations, Assessment,
Development, and Evaluation) of scientific evidence level of the
publications of the American College of Chest Physicians (ACCP), and of the
recently published level of recommendation.11
The levels of scientific evidence were characterized as A (strong
evidence), B (moderate evidence), and C (low or very low evidence) according to
the study design, the consistency of the results, and the clarity of the
evidence to answer clinical questions. This system was chosen for its
simplicity, transparency, explicitness, and consistency with the current
methodological approach for the development of evidence-based good clinical
practice. The recommendations were labeled according to the balance between
risk, benefit, social and epideÂmiological importance, and, in some cases,
cost. Recommendations can be level 1 (mandatory) or level 2 (doubtful). For
example, a 1A recommendation is a mandatory recommendation with strong
scientific evidence, while a 2C recommendation is one with low scientific
evidence, and is considered doubtful11.
The recommendations in response
to each question were subjected to the agreement of a panel of nine specialists
(four allergists and five pulmonologists) using the RAND/ UCLA modified Delphi
consensus methodology.12-13 AgreeÂment on
a recommendation was reached if a 75 % consensus was achieved. Each question
and its recommendation were discussed in virtual meetings by the panel of
specialists. If an agreement was not reached in the first round, a second round
was conducted after a review of the literature and proposals one week later.
All the recommendations reached a consensus exceeding 75 % within two rounds.
QUESTIONS AND RECOMMENDATIONS
About adherence
The World Health Organization
(WHO) defines adherence as âthe extent to which a patientâs use of
medication corresponds to the prescribed regimenâ.14
Patient behavior regarding treatment adherence is complex and
diverse. It is widely recognized that non-adherence is very common in patients
with asthma (30-70 %) and is motivated by numerous factors.1-3,9-10 The concept of poor adÂherence primarily
applies to the underutilization of daily preventive treatment.15-21 It is evident that poor adherence leads to an
increase in morbidity, mortality, and use of healthcare resources. The
psychosocial factors of the patient, inherent to the disease itself, the
doctor-patient relationship, and access to medications have been extensively
determined in the studies15-21.
In the different defiÂnitions of âpoorly controlled asthma,â assessing adherence
problems and addressing them before labeling a patient as having severe asthma
is a mandatory step in the recommendations of differÂent international and
national guidelines.1-3,
8-10 In
clinical studies, it is necessary to ensure treatment adherence more than 80 %
of the time, and this is achieved not only through patient self-reporting of
medication intake but also by counting the doses of the drugs under
investigation and using electronic dosing devices during each visit or through
telemedicine. Therefore, it is likely that better asthma control can be
achieved in these patients solely by improving adherence.21
Educating asthmatic patients is
essential and recommended in every A evidence guideline as regards its benefits
in reducing morbidity and improving adherence to treatment and follow-up.1-3,9,22-23
Due to its relevance, the initial
questions about different interventions to enhance adherence and their
respective recommendations were grouped together.
1. Question: What is the efficacy
of adding a written treatment plan to the routine care for adult outpatients
with asthma?
Justification:
All the international and local
guidelines agree on the need for providing an asthma patient with a written
treatment plan, despite its challenging implementation1-3,8-9,22-23 and the limited scientific evidence
regarding the positive effects of having this tool in asthma management.
On one hand, having a written
plan contribÂutes to the inclusion of the patient as an active participant in
their treatment and as the central figure in their own condition. Thus, it is
expected that knowledge of the treatment and action plan allows the patient to
have better control over the disease, providing them with more tools to promptly
request a consultation if necessary. Additionally, a written plan raises
awareness of the disease among the patients, reducing the undesirable effects
caused by the low risk perÂception associated with asthma. At this point, the
importance of a written treatment plan is significant, as individuals diagnosed
with asthma often underestimate their symptoms and the potentially fatal
outcomes, especially in cases of poorly controlled asthma. 6-8
On the other hand, the practical implementation
of a written treatment plan is considered highly feasible due to its low cost,
and is also beneficial for healthcare personnel involved with asthma patients
and caregivers of high-risk asthmatic groups.1-3,8-9,22-24
Lastly, a tool like the written
treatment plan can help enhance adherence to asthma treatments, which is
generally low, and its improvement is top priority.
Recommendation
The use of personalized
management plans is recommended because of their low cost, although the
benefits of using them in combination with standard treatment have an uncertain
impact on exacerbations, asthma control, and improvement in quality of life (1C).
2. Question Should
remote electronic reÂsources be used instead of the routine care for adult
outpatients with asthma?
Justification
There is significant
variability in the scientific evidence regarding the use of remote electronic
resources for enhancing treatment adherence in asthma patients. The uncertainty
arises from methodological heterogeneity in the studied popuÂlations and
measurement methods, both of which affect the variability of the effect size.25
More specifically,
there is high certainty for treatment adherence evaluated through electronic
monitoring, low certainty for adherence evaluated through self-report25,
and very low certainty for the adherence evaluated through pharmacy aerosol
refill. So, evidence results are inconsistent and inconclusive regarding the
actual effectiveness of using remote electronic resources to enhance adherence
to asthma treatment.
Recommendation
Broad implementation
of remote elecÂtronic resources to enhance adherence in patients with asthma is
not recommended (2B).
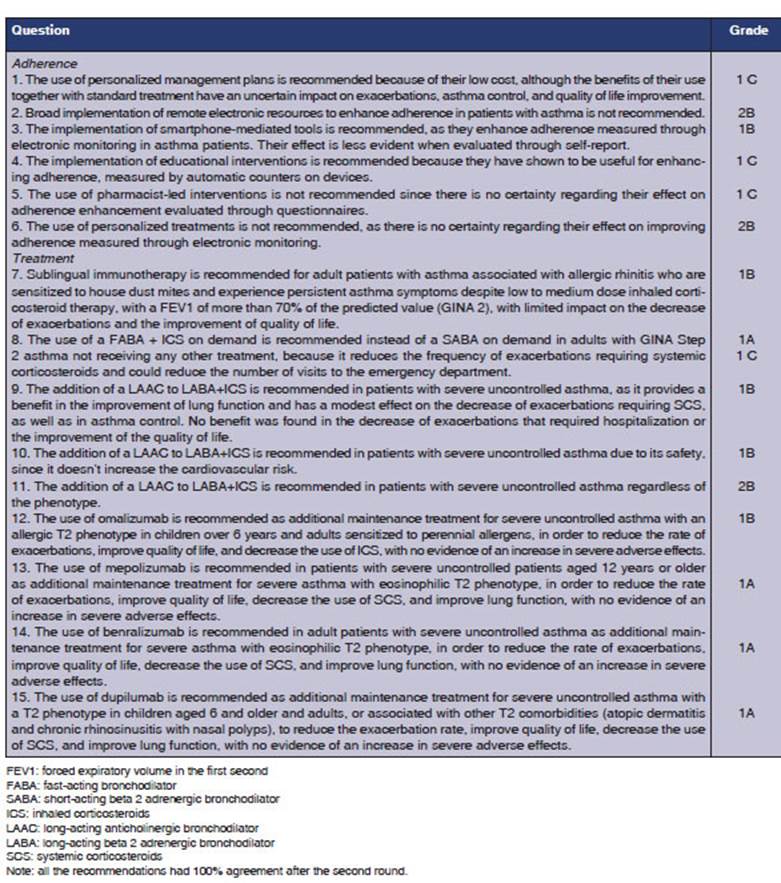
3. Question. Should smartphone-based
interventions be used instead of the routine care for adult patients with
asthma?
Justification
The certainty about
the positive relationship beÂtween the use of mobile phone-mediated tools and
the improvement in adherence is high. However, certainty is moderate for the
adherence evaluated through self-report.26
Recommendation
The implementation of
mobile phone-mediated tools is recommended as they enhance adherence when
measured through electronic monitoring in asthma patients. Their effect is less
evident when evaluated through self-report (1B).
4. Question. Should
education be used as an intervention to promote adherence instead of the
routine care for adult outpaÂtients with asthma?
Justification
As previously
mentioned, education is essential in the treatment and follow-up of asthma paÂtients.1-3,9,22-23,27 In fact, the set of educational conÂtent
directly or indirectly favors adherence. Among the relevant content that should
not be omitted, there is the correct diagnosis, guidelines for early
recognition of exacerbations and how to respond to them, tools to recognize
different types of conÂtrol and rescue pharmacological treatments, the
importance of adherence, as well as the relevance of proper usage techniques,
of reducing adverse events, seeking medical consultations promptly, and
managing comorbidities correctly.1-3,9,22-23,27
Recommendation
The implementation of
educational interÂventions is recommended because they have shown to be useful for
enhancing adherence, measured by automatic counters on devices (1C).
5. Question. Should
pharmacist-led inÂterventions be used to promote adherence instead of the
routine care for adult outpaÂtients with asthma?
Justification
Problems with
adherence to treatment and follow-up are quite common in asthma patients, and
this can be attributed to multiple factors, as mentioned earlier. Many studies
have invesÂtigated various types of interventions of pharÂmaceutical personnel
to evaluate the beneficial impact on adherence, but yielded inconclusive
results. The certainty to improve adherence is very low for pharmacy aerosol
refill and low for self-report.28
Recommendation
The use of
pharmacist-led interventions is not recommended, since there is no cerÂtainty
regarding their effect on adherence enhancement evaluated through questionÂnaires
(1C).
6. Question. Should
personalized treatÂment be used as an intervention to promote adherence instead
of the routine care for adult outpatients with asthma?
Justification
Due to contradictory
published data regarding the use of mobile personalized action plans versus
written action plans and the risk of bias, it is not clear at this time whether
one format of action plan is superior to the other for both adolescents and
adults.27
Recommendation
The use of
personalized treatments is not recommended, as there is no certainty regarding
their effect on improving adherÂence measured through electronic monitorÂing
(2B).
About the treatment
7. Question Should pharmacological treatÂment + sublingual immunotherapy
(SL) be used instead of pharmacological treatment or placebo in adult patients
diagnosed with allergic asthma?
Justification
The latest edition of
the Global Initiative for Asthma (GINA), states that specific immunoÂtherapy
(SIT) for allergens can be a treatment option where allergy plays a prominent
role, including asthma with allergic rhinoconjunctivitis.1 There are
two forms of SIT: sublingual (SLIT) and subcutaneous (SCIT). While modest
effects were identified in a systematic review of SLIT for asthma in adults and
children, these effects are predominantly limited to oral and gastrointestinal
symptoms.29 As with any treatment, the
potential benefits of SLIT for individual patients should be weighed against
the risk of adverse effects and the cost for both the patient and the
healthcare system.1
On the other hand, in
GEMA 2022, reference is made to SCIT with allergenic extracts as an effecÂtive
treatment for well-controlled allergic asthma at low or moderate levels of
treatment (therapeutic steps 2 to 4), provided that clinically relevant IgE-mediated sensitization to common aeroallergens has been
demonstrated, well-characterized and standardized extracts are used, and the
use of complex mixtures is avoided.1-2,30-33 However, many patients
with mild intermittent asthma (step 1) concurrently suffer from moderate or
severe alÂlergic rhinitis, which justifies the prescription of immunotherapy.34
SCIT should not be prescribed to patients with severe uncontrolled
asthma, as its effectiveness is not well-documented and there is a high risk of
suffering severe, even life-threatening adverse reactions.33,35
Therefore, it should be prescribed by specialized physicians with experiÂence
in this type of treatment and administered in facilities equipped with basic
measures for the immediate treatment of a potential severe adverse reaction.
The search for safer and more convenient alternatives for the patients has
stimulated the study of the effectiveness of SLIT. Most clinical trials that
demonstrated clinical efficacy have used well-characterized extracts at doses
much higher than those typically used in SCIT. The tolerance profile of
sublingual immunotherapy is optimal, with no fatal reactions reported.34,36
When sublinÂgual immunotherapy (SLIT) in oral lyophilized form for dust mites
is added to the controlled maintenance pharmacological treatment, it is caÂpable
of reducing the number of moderate to severe exacerbations and improving
disease control, with a very good safety profile.37 Therefore, its
use is recommended in adult patients with moderately controlled or partially
controlled asthma.34 If variÂous immunotherapy alternatives are
available, priÂority should be given to those that have the status of registered
medicines with well-established effiÂcacy, safety, and quality. At the moment,
there are no comparative studies on the cost-effectiveness of immunotherapy
versus conventional pharmaÂcotherapy, and sure enough, such studies will not be
conducted, as the complexity of their design makes them poorly viable. However,
specific imÂmunotherapy (SIT), in addition to controlling the manifestations of
the disease, offers several adÂditional advantages over pharmacotherapy. These
include maintaining clinical benefits obtained until several years after
treatment cessation, reducing the risk of developing asthma in patients with
allergic rhinitis, or preventing the development of new sensitizations in
mono-sensitive patients 38-41. Furthermore, allergen immunotherapy has
a unique immunological justification, as it tailors the approach to an
individualâs specific IgE spectrum and modifies the
natural course of the disease, with persistent efficacy after treatment
completion. From this perspective, allergen immunotherapy (AIT) should
currently be considered a prototype of Precision Medicine.42
Recommendation
Sublingual
immunotherapy is recommendÂed for adult patients with asthma associated with
allergic rhinitis who are sensitized to house dust mites and experience persistent
asthma symptoms despite low to medium dose inhaled corticosteroid therapy, with
a FEV1 (forced expiratory volume in the first second) of more than 70 % of the
predicted value (GINA 2), with limited impact on the decrease of exacerbations
and the improveÂment of quality of life (1B).
8. Question. Should a
fast-acting bronchoÂdilator (FABA) + inhaled corticosteroids (ICS) on demand be
used instead of a short-acting beta-2
adrenergic bronchodilator (SABA) on demand in adults with GINA Step 2 asthma?
Justification
Based on new clinical
information, in 2021 major international guidelines made changes to asthma
treatment strategies.1-2 The recommenÂdation for fixed-dose inhaled corticosteroids
in GINA Step 2 is still the treatment of choice, as it allows for better
disease control and has consistent accessibility within the healthcare system.1
Itâs important to note that the controlled, prospecÂtive, double-blind studies
that have been analyzed were conducted with the fixed-dose combination of
budesonide-formoterol.43-48 This treatment strategy has shown a high
level of evidence in the decrease of exacerbations that require the use of
corticosteroids (CS). However, it does not have the same level of evidence for
other variables such as emergency department visits, improved quality of life,
asthma control, and improved lung function.49
While the analyzed information
may not strictly apply to patients included in GINA Step 1, it is understood
that this strategy could be applied to such patients, with a lower level of
evidence. As a strong recommendation in favor of using the budesonide-formoterol combination on demand, which is available in our
country, the goal is to reduce the frequency of exacerbations that reÂquire
systemic corticosteroids, likely leading to a decrease in the number of visits
to the emergency department.
Recommendation
The use of a fast-acting
bronchodilator + inhaled corticosteroids (FABA + ICS) on demand is recommended
instead of a short-acting beta-2 adrenergic bronchodilator (SABA) on demand in
adults with GINA Step 2 asthma not receiving any other treatment, because it
reduces the frequency of exacerÂbations requiring systemic corticosteroids
(1A), and could reduce the number of visits to the emergency department (1C).
9. Question. Should long-acting
antichoÂlinergic bronchodilators (LAACs) + ICS + long-acting beta-2 adrenergic
bronchodilaÂtors (LABA) be used instead of LABA + ICS for adult patients with severe
uncontrolled asthma?
Justification
Anticholinergic bronchodilators
were among the first pharmacological groups used to treat asthma, as a natural
component of belladonna. Beyond their bronchodilator action, long-acting
antichoÂlinergics have anti-inflammatory effects
through both neuronal and non-neuronal route, acting on inflammatory cells and
molecules.50 Tiotropium is the LAAC with
the largest amount of clinical information and has been studied in children,
adoÂlescents, and adults.51 Two other LAACs (glycopyrÂronium
and umeclidinium) have been investigated in the
CAPTAIN, IRIDIUM, TRIMERAN, TRIGÂGER, and ARGON studies, evaluating the
clinical impact of the triple therapy in a single inhaler containing three
pharmacological groups (LAAC, LABA, and inhaled corticosteroids) in patients
with moderate and severe asthma not controlled with ICS/LABA.52-55
There are some differences in the evidence related to the duration of action of
each drug, as well as the quantity and quality of available studies and the
specific combinations of LABA+LAAC+ICS.
In an extensive review that
included some studies with all three LAACs (11,894 children and adults; mean
age: 52 years [range, 9-71 years]; 57.7 % women), the main objectives assessed
were severe exacerbations, asthma control (measured by the Asthma Control
Questionnaire, ACQ-7), quality of life (measured using the Asthma Quality of
Life Questionnaire, AQLQ), mortality, and adÂverse events.56 The
results obtained demonstrated (with high certainty) that triple therapy versus
dual therapy (LABA+ICS) in a single device once-daily was significantly
associated with improved lung function (high certainty) and reduced risk of
exacerbations requiring systemic corticosteroids (SCS) (moderate certainty), or
hospitalization (low certainty).56 There were no significant
differences regarding the quality of life (high certainty of the evidence) or
mortality (high certainty of the eviÂdence) between dual and triple therapy.56
Triple therapy was significantly
associated with an increase in dry mouth and dysphonia, and for serious adverse
events, there was no difference between the groups, including cardiovascular
events (moderate certainty of the evidence).52-55,57-61
One of the benefits of a fixed
triple therapy verÂsus an open one could be better treatment adherÂence,
as it would reduce the number of inhalers a patient needs to use, as well as
the number of doses. Despite these results and other previous studies with
another fixed triple therapy (TRIÂMARAN and TRIGGER), more studies are needed
to confirm these improvements, especially with regard to exacerbations.53-55
Most safety studies were
conducted in patients with COPD (higher mean age, and higher number of
concomitant diseases of greater severity). In asthma, only one study on
cardiovascular effects has moderate certainty of the evidence.61-62
According to all asthma
management guideÂlines, patients with severe asthma should be pheÂnotyped.63-64
Regarding the therapeutic approach in this stage, the efficacy of the LAACs is
independent of the asthma phenotype, irrespective of the eoÂsinophilia degree
and the fraction of exhaled nitric oxide (FeNO).65-66
The addition of LAACs could be considered for patients with persistent
bronchial obstruction, symptomatic patients, and patients who are not frequent exacerbators (low certainty of the evidence).52-55
The GINA guideline recommends
their use in patients who continue to have exacerbations despite intensive
treatment with two controllers (inhaled corticosteroids and LABA), at step 4 or
5.1 The GEMA (GuÃa EspaÃąola para
el Manejo del Asma)
guideline suggests the use of LAACs from step 4 and 5 in combination with ICS
and LABA.2 The ATS/ERS (American Thoracic Society/EuroÂpean
Respiratory Society) guideline recommends them in children, adolescents, and
adults with severe uncontrolled asthma regardless of the GINA step 4/5
controller treatment.63
Recommendation
The addition of a LAAC to
LABA+ICS is recommended in patients with severe unconÂtrolled asthma, as it
provides a benefit in the improvement of lung function and has an effect on the
decrease of exacerbations reÂquiring corticosteroids, as well as in asthma
control. No benefit was found in the decrease of exacerbations requiring
hospitalization or the improvement of quality of life (1B).
10. Question Is
it safe to use LAAC+ICS+LABA in adult patients with severe uncontrolled asthma?
Justification
The rationale for this question
is based on question 9.
Recommendation
The addition of a LAAC to
LABA+ICS is recommended in patients with severe uncontrolled asthma due to its
safety, since it doesnât increase the cardiovascular risk (1B).
11. Question Should LAAC+LABA+ICS
be used according to the phenotype for adult patients with severe uncontrolled
asthma?
Justification
The rationale for this question
is based on question 9.
Recommendation
The addition of a LAAC to
LABA+ICS is recommended in patients with uncontrolled severe asthma regardless
of the phenotype (2B).
Biologics
Severe asthma constitutes 3 to 5
% of the population with asthma. It is characterized by the persistence of
symptoms, higher number of visits to emergency rooms or unscheduled outpatient
consultations, more hospitalizations, an increased use of rescue medication,
systemic corÂticosteroids, antibiotics, and the resulting impact on the
increased use of healthcare resources and increased mortality.63-64 Severe asthma represents a heterogeneous syndrome with
multiple clinical variants. Over the past two decades, it has been intensely
studied, and different phenotypes have been defined.67-69
Establishing the asthma phenoÂtype in patients with severe uncontrolled asthma
is part of the diagnosis and evaluation of these individuals, since it can lead
to differential treatÂment and have prognostic implications. 63-64,67-69 Two inflammatory phenotypic patterns have been
defined: T2-high (present in allergic and eosinoÂphilic
asthma) and non-T2, also called T2-low. Both T2-high phenotypes often show some
degree of overlapping. The fraction of exhaled nitric oxide, eosinophilia, and IgE are good biomarkers for the T2-high phenotype. Allergic
T2 asthma represents 40-50 % of severe asthma and has an atopic basis
orchestrated by the activation of T helper type 2 cells (Th2), the production
of interleukins IL-4, IL-5, and IL-13, and isotype
switching in B lymÂphocytes towards IgE production. Eosinophilic T2 asthma represents more than 25 % of severe
asthma and is characterized by the presence of eosinophils
in bronchial biopsies and sputum, even in patients receiving high doses of
glucocorticoids. It may be associated with chronic rhinosinusitis
and nasal polyps.63-64,67-69
The following questions and their
respective recommendations are related to the use of biologÂics in severe
asthma.
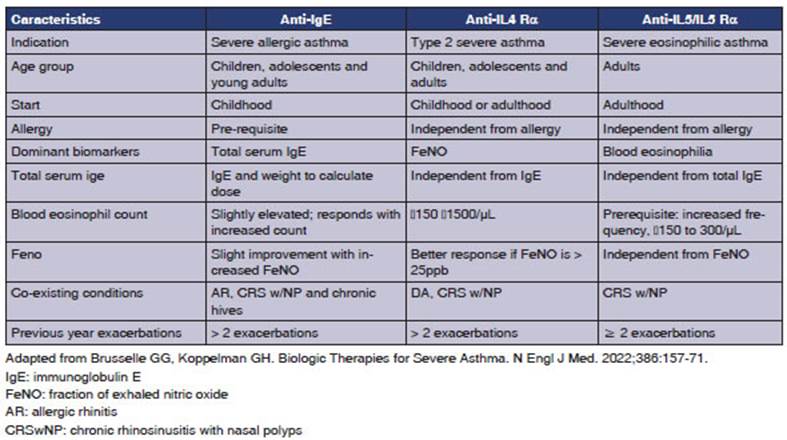
12. Question. Should omalizumab be used instead of placebo in patients with
severe uncontrolled asthma with a T2 phenotype?
Justification
Omalizumab is a humanized monoclonal anti- IgE antibody
(Mab) that binds to free IgE,
preÂventing its attachment to mast cell and basophil receptors, and it can also
reduce IgE receptors on effector cells.63-64
Omalizumab is indicated for type 2 allergic asthma with total IgE values between 30-1,500 IU. The dosage varies
depending on the IgE level and body weight.63-64
The quality of the evidence is
high, the magÂnitude of beneficial effects is moderate, and the magnitude of
adverse events (AEs) is low. Therefore, the benefit-risk ratio favors the use
of omalizumab.63-64,71-76
The evidence is moderate
regarding the decrease of exacerbations requiring SCS and the improveÂment of
FEV1.63-64,71-76 The evidence is low for
the decrease of exacerbations requiring emergency care and hospitalization and
for the improvement of the ACQ.63-64,71-76
There are no cost-effectiveness studies
in our country, but despite the high cost, most probably the benefit-risk ratio
favors the use of the drug.
Recommendation
The use of omalizumab
is recommended as additional maintenance treatment for severe uncontrolled
asthma with an allergic T2 phenotype in children over 6 years and adults
sensitized to perennial allergens, in order to reduce the rate of
exacerbations, improve quality of life, and decrease the use of SCS, with no
evidence of an increase in severe adverse effects (1B).
13. Question. Should mepolizumab be used instead of placebo in adult patients
with severe uncontrolled asthma with a T2 phenotype?
Justification
Mepolizumab and reslizumab are both IL-5 inhibitors, but
only mepolizumab is commercially available in our
country.70
The quality of the evidence is
high, the magnitude of beneficial effects is moderate, and the magnitude of
adverse events is low. Therefore, the benefit-risk ratio favors the use of
mepolizumab.70,77-81
The evidence is of high quality
regarding the decrease of exacerbations requiring SCS and those requiring
emergency care or hospitalizaÂtion.70,77-81
The evidence is moderate regarding the improvement of the ACQ and the
improvement of FEV1.70,77-81
There are no cost-effectiveness
studies in our country, but despite the high cost, most probably the
benefit-risk ratio favors the use of the drug.
Recommendation
The use of mepolizumab
is recommended in patients with severe uncontrolled asthÂma aged 12 years or
older as additional maintenance treatment for severe asthma with eosinophilic T2 phenotype, in order to reduce the rate of
exacerbations, improve quality of life, decrease the use of SCS, and improve
lung function, with no evidence of an increase in severe adverse effects (1A).
14. Question. Should benralizumab be used instead of placebo in adult patients
with severe uncontrolled asthma with a T2 phenotype?
Justification
Benralizumab is an inhibitor of the IL-5 reÂceptor α .70 The quality of the evidence is
high, the magnitude of beneficial effects is moderate, and the magnitude of
adverse events is low.82-87 Therefore, the benefit-risk ratio favors
the use of benralizumab.82-87
The evidence is high regarding
the decrease of exacerbations requiring SCS and the improvement of FEV1.82-87
The evidence is of high quality regardÂing the
improvement of quality of life (ACQ) and the decrease of exacerbations
requiring emergency care or hospitalization.82-87
There are no cost-effectiveness
studies in our country, but despite the high cost, most probably the
benefit-risk ratio favors the use of the drug.
Recommendation
The use of benralizumab is recommended in adult patients with severe
uncontrolled asthma as additional maintenance treatÂment for severe asthma with
eosinophilic T2 phenotype, in order to reduce the
rate of exacerbations, improve quality of life, decrease the use of SCS, and
improve lung function, with no evidence of an increase in severe adverse
effects (1A).
15. Question. Should dupilumab be used instead of placebo in patients with
severe uncontrolled asthma with a T2 phenotype?
Justification
Dupilumab is an inhibitor of the IL-4 receptor α subunit, which interferes with the action of both IL-4 and IL-13.88
The quality of the evidence is
high, the magÂnitude of beneficial effects is moderate, and the magnitude of
adverse events is low.88-93 Therefore, the benefit-risk ratio favors
the use of dupilÂumab.88-93
The evidence is of high quality
regarding the decrease of exacerbations requiring SCS and those requiring
emergency care or hospitalization, and also regarding the improvement in ACQ
and the FEV1.88-93
There are no cost-effectiveness
studies in our country, but despite the high cost, most probably the
benefit-risk ratio favors the use of the drug.
Recommendation
The use of dupilumab
is recommended as additional maintenance treatment for severe uncontrolled
asthma with a T2 phenoÂtype in children aged 6 and older and adults, or
associated with other T2 comorbidities (atopic dermatitis and chronic rhinosinusÂitis with nasal polyps, CRSw/NP),
to reduce the exacerbation rate, improve quality of life, decrease the use of
SCS, and improve lung function, with no evidence of an inÂcrease in serious
adverse effects (1A).
GENERAL CONCLUSION REGARDING BIOLOGICS (TABLE 3)
Severe uncontrolled asthma is
associated with a reduced quality of life, increased exacerbations, hospital
admissions with frequent use of systemic corticosteroids, and elevated death
risk.63-64 PhenoÂtyping patients with
severe uncontrolled asthma (SUA) is necessary to prescribe the precise biologic
therapy for each phenotype.63-64
Biologics targeting type 2
inflammation have shown improvement in disease control when used as additional therapy
alongside maintenance treatÂment in patients with SUA (Step 5 according to
GINA, Step 6 according to GEMA).1-2,63-64,94
FINAL CONCEPTS
The modified Delphi methodology
is a well-defined technique for reaching a consensus among specialÂists in areas
of uncertainty, and it is particularly useful for making decisions in medical
situations where scientific evidence is scarce or nonexisÂtent.13-14
One of the strengths of this document is that it achieved 100 % agreement among
the participants within two rounds. Another strength
is that the specialists were selected by the two scientific societies for their
expertise in the topics being discussed. The Delphi technique suggests that the
participation of up to twelve specialists is sufficient and recommended.13-14
All authors had the opportunity to vote freely and
express their opinions during discussion moments. This manuscript also has some
limitations. The absence of participation from clinical physicians may, to some
extent, limit the perspective regarding adherence or asthma management,
especially in milder forms of the disease. Another limitation is that the
specialistsâ opinions, as reflected in the selection of the supporting
literature or their own experience in the field, may not encompass all the
published evidence in the area. Furthermore, it has limited temporal validity
and may change with the emergence of new scientific information. It should be
interpreted rationally and complemented in the future with further research,
especially within the context of areas of greater uncertainty.
In conclusion, this document
provides recomÂmendations based on expert opinion and grounded in scientific
evidence with regard to the importance of enhancing adherence to treatment and
follow-up through different asthma management strateÂgies, especially given the
frequent poor control of asthma in our country.7-8 It also provides updated recommendations on the critical
aspects of the treatment of mild to severe asthma.
KEY POINTS
Current knowledge
Despite the advances in asthma
management and preÂventive treatment that improve quality of life and reduce
morbidity and mortality, our country still has poor asthma control and an
unacceptable rate of hospitalizations and mortality.
Contributions of the article to current knowledge:
Specialists from two medical
societies committed to taÂking actions to improve asthma control in our country
have made locally adapted recommendations in various critical aspects of asthma
management and treatment.
Acknowledgement
The authors thank the
collaboration of Dr. Alejandro Videla and DamiÃĄn Silva and Vanesa Kirchuk as methodological advisors.
Conflict of interest
This manuscript was not funded by
any pharmaceutical company, but rather was a peer education project initiated
by both sponsoring scientific societies.
Pablo Moreno: no conflict of
interest.
Susana Luhning:
no conflict of interest.
Anahà YaÃąez: clinical research investigator for
GSK, AstraZeÂneca, Sanofi Chiessi,
Novartis, MDS, Roche, Faes, TEVA, Avillon,
Bayer, Sanofi Gynzene. Medical advisor for GSK, AstraZeneca, Eurofarma,
Sanofi Genzyme, Novartis. Continuing
medical eduÂcation activities for Sanofi Genzyme and
GSK.
Ana Stock: clinical research
investigator for AstraZeneÂca, Sanofi, GSK, Novartis,
Chiesi, ZambÃģn, Bristol,
Bayer, MSD, and Roche. Continuing medical education activities for AstraZeneca Sanofi, GSK, ELEA Phenix, Novartis. Medical advisor for GSK,
AstraZeneca, Sanofi and ELEA Phoenix.
Gabriel Gattolin:
speaker and clinical research investigaÂtor for AstraZeneca, Sanofi, Novartis, GSK, AbbVie,
Roche, Amgen, Lilly, Pfizer, TEVA Pharma, Chiessi, and Takeda.
Walter Mattarucco:
speaker in continuing medical eduÂcation activities for AstraZeneca. Researcher in asthma for Novartis, Sanofi,
AstraZeneca.
Martin Maillo:
clinical research investigator for: GSK, Novartis, Astra-ZÃĐneca,
Forrest, Pearl, Chiesi, Sanofi,
RoÂche, Janssen, TRI, Insmed and Zambon.
Has received fees as a speaker or advisor from: GSK,
AstraZeneca, Novartis, and Sanofi/Genzyme.
Marcos HernÃĄndez: continuing
medical education activities for ELEA, Boehringer Ingelheim, AstraZeneca, GSK, Tuteur.
MartÃn Sivori:
continuing medical education activities for ELEA, GSK, TEVA, AstraZeneca.
REFERENCES
1. Global Initiative
for Asthma. Difficult to treat & Severe Asthma in adolescent and adult
patients: Diagnosis and Treatment. 2022. Acceso el 2 de febrero de
2023 en www.ginasthma.org.
2. Alobid I, Ãlvarez RodrÃguez C, Blanco
Aparicio M, et al. GEMA 5.2. GuÃa EspaÃąola para el
manejo del asma. ISBN: 978-84-19069-13-9. Acceso el 2 de febrero de 2023 en
www.gema.com.
3. British Thoracic
Society. Sign 158: British Guideline on the management of asthma. 2019.
4. Forno E, Gogna
M, Cepeda A, et al. Asthma
in Latin America. Thorax
2015;70:898-905.
https://doi.org/10.1136/thoraxjnl-2015-207199
5. Arias S, Neffen H, Bossio JC, et al.
Prevalence and features of asthma in young adults in urban areas of Argentina.
Arch Bronconeumol 2018;54:134-9.
https://doi.org/10.1016/j.arbres.2017.08.021
6. Neffen H, Fritscher C, Cuevas
Schacht F, et al. Asthma control in Latin America: the Asthma Insights and RealÂity
in Latin America (AIRLA) Survey. Rev Panam Salud Publica 2005;17:191-7.
https://doi.org/10.1590/S1020-49892005000300007
7. Maspero JF, Jardim
JR, Aranda A, et al. Insights,
attitudes and perceptions about asthma and its treatment: findings from a
multinational survey of patients from Latin America. World Aller
Org J 2013;6:19.
https://doi.org/10.1186/1939-4551-6-19
8. Colodenco D, Neffen H, Baena-Cagnani C, et al. Recomendaciones para el
diagnÃģstico y tratamiento del asma de difÃcil control
(ADC). Rev Am Med Resp
2006:15-36.
9. Yang CL, Hicks EA,
Mitchell P, et al. Canadian Thoracic Society 2021 Guideline update: diagnosis
and management of asthma in preschoolers, children and adults. Can J Respir Crit Care Sleep Med 2021;5:348-61. https://doi.org/10.108 0/24745332.2021.1945887
10. Programa Nacional de prevenciÃģn y control de las enfermedades
respiratorias crÃģnicas. Protocolo de OrientaciÃģn para el diagnÃģstico y manejo
del asma en adultos. Ministerio de Salud de NaciÃģn. DirecciÃģn de PromociÃģn de
Salud y Enfermedades No Transmisibles. 2015.
11. Guyatt G, Gutterman D, Baumann
MH, et al. Grading strength of recommendations and quality of evidence in
clinical guidelines: report from an American College of Chest Physicians Task
Force. Chest. 2006;129:174-81.
https://doi.org/10.1378/chest.129.1.174
12. The Delphi
Method. Techniques and Applications Edited by Harold A. Linstone.
Portland State University Murray Turoff.
New Jersey Institute of Technology 2002 Murray Turoff and Harold A. Linstone.
13. Fitch K,
Bernstein SJ, Aguilar MD, et al. The RAND/UCLA appropriateness method userâs
manual. Acceso el 13 de julio de 2023 en: https://www.rand.org/content/dam/rand/pubs/monograph_reports/2011/MR1269.pdf
14. Osterberg L, Blaschke T.
Adherence to medication. N Engl J Med 2005;
353:487-97. https://doi.org/10.1056/NEJMra050100
15. Gillissen A. Patientâs adherence in asthma. J Physiol Pharmacol 2007;58: 205-222.
16. Haynes RB, Ackloo E, Sahota N, McDonald HP,
Yao X. Interventions for enhancing medication adherence. CoÂchrane Database Syst Rev 2008:CD000011.
https://doi.org/10.1002/14651858.CD000011.pub3
17. Bosley CM, Parry DT, Cochrane GM. Patient compliance with
inhaled medication: does combining beta-agonists with corticosteroids improve
compliance? Eur Respir J
1994;7:504-9.
https://doi.org/10.1183/09031936.94.07030 504
18. Sackett DL, Snow JC. The magnitude of
compliance and non-compliance. In: Haynes RB, Taylor WD, Sackett DL, editors. Compliance in health
care. Baltimore: Johns HopÂkins University Press; 1979. pp. 11-22.
19. Gamble J,
Stevenson M, McClean E, Heaney LG. The
prevaÂlence of nonadherence in difficult asthma.
Am J Respir Crit Care Med
2009;180:817-22.
https://doi.org/10.1164/rccm.200902-0166OC
20. Boslev Barnes C, Suppli Ulrik C. Asthma and adherence to inhaled corticosteroids:
current status and future perspecÂtives. Respir Care
2015;60:455-68. https://doi.org/10.4187/respcare.03200
21. Costello RW, Cushen B. Looking back to go forward: adherence to inhaled
therapy before biologic therapy in severe asthma Eur Respir J 2020;55:20000954.
https://doi.org/10.1183/13993003.00954-2020
22. Gibson PG, Powell
H, Coughlan J, et al. Limited (inforÂmation only) patient education programs
for adults with asthma. Cochrane Database Syst Rev
2002:CD001005. https://doi.org/10.1002/14651858.CD001005
23. Powell H, Gibson
PG, Options for self-management education for adults with asthma. Cochrane
Database Syst Rev 2003:CD004107.
https://doi.org/10.1002/14651858.CD004107
24. Gatheral TL, Rushton A, Evans DJ, Mulvaney
CA, Halcovitch NR, Whiteley
G, Eccles FJ, Spencer S. PersonalÂized asthma action
plans for adults with asthma. CoÂchrane Database Syst
Rev 2017:CD011859. https://doi.org/10.1002/14651858.CD011859.pub2
25. Jeminiwa R, Hohmann L, Qian J, Garza K, Hansen R, Fox BI. Impact of eHealth on medication adherence among patients with asthma:
A systematic review and meta-analysis. Respiratory Medicine 2019;149:59-68. https://doi.org/10.1016/j.rmed.2019.02.011
26. Murphy JA, Heisser JM, Montgomery M. Evidence-Based Review of
Smartphone Versus Paper Asthma Action Plans on Asthma
Control. J Pharm Technol 2019;35:126-34.
https://doi.org/10.1177/8755122519830446
27. Normansell R, Kew KM, Stovold E.
Interventions to imÂprove adherence to inhaled steroids for asthma (Review).
Cochrane Database of Systematic Reviews; 2017:CD012226.
https://doi.org/10.1002/14651858.CD012226.pub2
28. Jia X. Effect of pharmacist-led interventions on medication
adherence and inhalation technique in adult patients with asthma or COPD: A
systematic review and meta-analysis. J Clin
Pharm Ther. 2020;45:904-7.
https://doi.org/10.1111/jcpt.13126
29. Fortescue R, Kewk KM, Leung ST, Shiu T. Sublingual immunotherapy for asthma. Cochr Database Syst Rev 2020;9:CD011293. https://doi.org/10.1002/14651858.CD011293.pub3
30. Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma.
Cochrane Database Syst Rev. 2010;(8):CD001186.
https://doi.org/10.1002/14651858.CD001186.pub2
31. Dhami
S, Kakourou A, Asamoah F,
et al. Allergen immunoÂtherapy for allergic asthma: a systematic review and
meta-analysis. Allergy. 2017;72:1825-48.
https://doi.org/10.1111/all.13208
32. Adkinson
NF Jr, Eggleston PA, Eney
D, al. A controlled trial of immunotherapy for asthma in
allergic children. N Engl J Med. 1997;336:324-31. https://doi.org/10.1056/NEJM199701303360502
33. Pitsios
C, Demoly P, Bilo MB, et
al. Clinical contraindications to allergen immunotherapy: an EAACI position
paper. Allergy 2015;70:897-909.
https://doi.org/10.1111/all.12638
34. Agache
I, Lau S, Akdis CA, et al. EAACI Guidelines on
Allergen Immunotherapy: House dust mite-driven allergic asthma. Allergy 2019;74:855-73. https://doi.org/10.1111/all.13749
35. Bernstein DI, Wanner M, Borish L, Liss GM, Immunotherapy Committee, American Academy of
Allergy, Asthma and Immunology. Twelve-year survey of fatal reactions to
allergen injections and skin testing: 1990-2001. J Allergy Clin
Immunol 2004;113:1129-36.
https://doi.org/10.1016/j.jaci.2004.02.006
36. Lin SY, Erekosima
N, Kim JM, Ramanathan M, et al. Sublingual
immunotherapy for the treatment of allergic rhinoconjunctivitis
and asthma: A systematic review. JAMA 2013;309:1278-88.
https://doi.org/10.1001/jama.2013.2049
37. Virchow JC, Backer V, Kuna P,
et al. Efficacy of a house dust mite sublingual allergen immunotherapy tablet
in adults with allergic asthma: A randomized clinical trial. JAMA.
2016;315:1715-25.
https://doi.org/10.1001/jama.2016.3964
38. Durham SR, Walker SM, Varga EM, et al. Long-term clinical efficacy of
grass-pollen immunotherapy. N Engl J Med. 1999;341:468-75. https://doi.org/10.1056/NEJM199908123410702
39. Jacobsen L, Niggemann B, Dreborg S, et al. The PAT investigator group. Specific immunotherapy has
long-term preventive effect of seasonal and perennial asthma: 10-year follow-up
on the PAT study. Allergy. 2007;62:943-8.
https://doi.org/10.1111/j.1398-9995.2007.01451.x
40. Kristiansen M, Dhami S, Netuveli G, et al.
Allergen immunotherapy for the prevention of allergy: A systematic review and
meta-analysis. Pediatr Allergy Immunol. 2017;28:18- 29. https://doi.org/10.1111/pai.12661
41. Pajno
GB, Barberio G, de Luca F, Morabito
L, ParmÂiani S. Prevention of new sensitizations in
asthmatic children monosensitized to house dust mite
by speÂcific immunotherapy. A six-year follow-up study.
Clin Exp Allergy 2001;31:1392-7. https://doi.org/10.1046/j.1365-2222.2001.01161.x
42. Canonica
GW, Bachert C, Hellings P,
Ryan D, Valovirta E, Wickman
M, De Beaumont O, Bousquet J. Allergen ImÂmunotherapy
(AIT): a prototype of Precision Medicine. World Allergy Organ J. 2015;8:31. https://doi.org/10.1186/s40413-015-0079-7
43. OÂīByrne PM, FitzGerald JM,
Bateman ED, et al. Inhaled combined budesonide-formoterol
as needed in mild asthma. N Engl J Med 2018;378:1865-76. https://doi.org/10.1056/NEJMoa1715274
44. Bateman ED, Reddel HK, OÂīByre PM PM, et al. As-needed budesonide-formoterol
versus maintenance budesonide in mild asthma. N Engl
J Med 2018;378:1877-87.
https://doi.org/10.1056/NEJMoa1715275
45. Beasley R, Holliday M, Reddel HK, et al. Controlled trial of budesonide-formoterol as needed for mild asthma. N Engl
J Med 2019;380:2020-30. https://doi.org/10.1056/NEJMoa1901963
46. Hardy J, Baggott
C, Fingleton J, et al. Budesonide-forÂmoterol
reliever therapy versus maintenance budesonide plus terbutaline
reliever therapy in adults with mild to moderate asthma (PRACTICAL): a 52-week,
open-label, multicenter, superiority, randomized controlled trial. Lancet 2019;394:919-28. https://doi.org/10.1016/S0140-6736(19)31948-8
47. Tanaka A, Ohta
S, Yamamoto M, et al. Tolerability of as-needed treatment with budesonide and formoterol combiÂnation in adult patients with mild asthma.
Am J Resp Crit Care Med
2017;195:A3199.
48. Haahtela
T, Tamminen K, Malmberg LP,
et al. Formoterol as needed with or without
budesonide in patients with intermittent asthma and raised NO levels in exhaled
air: A SOMA study. Eur Resp
J 2006;28:748-55.
https://doi.org/10.1183/09031936.06.00128005
49. Crossingham
I, Turner S, Ramakrishnan S, et al. Combination
fixed-dose beta agonist and steroid inhaler as required for adults or children
with mild asthma (Review). Cochrane Database of Systematic Reviews;
2021:CD13518. https://doi.org/10.1002/14651858.CD013518.pub2
50. Wessler
I, Kirkpatrick CJ. Acetylcholine beyond neurons: the non-neuronal cholinergic
system in humans. Brit J Pharmacol 2008; 154:1558-71.
https://doi.org/10.1038/bjp.2008.185
51. Halpin
D. Tiotropium in asthma: what is the evidence and how
does it fit in? World Allergy Org J 2016;9:29.
https://doi.org/10.1186/s40413-016-0119-y
52. Lee LA, Bailes
Z, Barnes N, et al. Efficacy and safety of once-daily single-inhaler triple
therapy (FF/UMEC/VI) versus FF/VI in patients with inadequately controlled
asthma (CAPTAIN): a double-blind, randomized, phase 3A trial. Lancet Respir Med 2021;9:69-84.
https://doi.org/10.1016/S2213-2600(20)30389-1
53. Kerstjens
HAM, Maspero J, Chapman KR, et al. Once-daily,
single-inhaler mometasone-indacaterol-glycopyrronium
versus mometasone-indacaterol or twice-daily
fluticasone-salmeterol in patients with inadequately
controlled asthma (IRIDIUM): a randomized, double-blind, controlled phase 3
study. Lancet Respir Med 2020;8:1000-12.
https://doi.org/10.1016/S2213-2600(20)30190-9
54.
Virchow JC, Kuna P, Paggiaro P, et al. Single inhaler extrafine triple therapy in
uncontrolled asthma (TRIMARAN and TRIGGER): two double-blind, parallel-group,
randomÂized, controlled phase 3 trials. Lancet 2019; 394:1737-49.
https://doi.org/10.1016/S0140-6736(19)32215-9
55.
Gessner C, Kornmann O, Maspero J, et al. Fixed dose
combiÂnation of indacaterol/glycopyrronium/mometasone furoate once-daily
versus salmeterol/fluticasone twice-daily plus tiotropium once-daily in patients with uncontrolled asthma:
A randomized, Phase IIIb, non-inferiority study
(ARGON). Respir Med 2020;170:106021.
https://doi.org/10.1016/j.rmed.2020.106021
56. Oba Y, Anwer
S, Maduke T, Patel T, Dias S. Effectiveness and
tolerability of dual and triple combination inhaler therapies compared with
each other and varying doses of inhaled corticosteroids in adolescents and
adults with asthma: a systematic review and network meta-analysis. Cochrane
Database Syst Rev 2022:CD013799.
https://doi.org/10.1002/14651858.CD013799.pub2
57. Peters SP, Kunselman SJ, Icitovic N, et al. Tiotropium bromide step-up therapy for adults with
uncontrolled asthma. N Engl J Med 2010;363:1715-26. https://doi.org/10.1056/NEJMoa1008770
58. Tan LD, Alismail
A, Ariue B. Asthma guidelines: comparison of the
National Heart, Lung, and Blood Institute Expert Panel Report 4 with Global
Initiative for Asthma 2021. Curr Op Pulm Med 2022;28:234-44.
https://doi.org/10.1097/MCP.0000000000000867
59. Kew KM, Dahri
K. Long-acting muscarinic antagonists (LAMA) added to combination long acting
beta2-agonists and inhaled corticosteroids (LABA/ICS) versus LABA/ ICS for
adults with asthma. Cochrane Database Sys Rev 2016:CD
011721. https://doi.org/10.1002/14651858.CD011721
60. Tian
JW, Chen JW, Chen R, Chen X. Tiotropium versus
placebo for inadequately controlled asthma: A meta-analysis. Respir Care 2014;59:654-66.
https://doi.org/10.4187/respcare.02703
61. Kerstjens
HA, Engel M, Dahl R, et al. Tiotropium in asthma
poorly controlled with standard combination therapy. N Engl
J Med 2012;367:1198-207.
https://doi.org/10.1056/NEJMoa1208606
62. Scosyrev
E, van Zyl-Smit R, Kerstjens H, et al. CardioÂvascular safety of mometasone/indacaterol and mometasone/indacaterol/glycopyrronium once-daily fixed-dose combinations in
asthma: pooled analysis of phase 3 trials. Respir Med
2021;180:106311.
https://doi.org/10.1016/j.rmed.2021.106311
63. Holguin F, Cardet JC, Chung KF, et al. Management of severe asthma: an European Respiratory Society/American Thoracic Society
guideline. Eur Respir J
2020;55:1900588.
https://doi.org/10.1183/13993003.00588-2019
64. Global Initiative for Asthma.
Difficult to treat & severe asthma in adolescent and adult patients:
diagnosis and management. 2018. Acceso el 14 de febrero de 2023 en
www.ginasthma.org.
65.
Iwamoto H, Yokoyama A, Shiota N, et al. Tiotropium bromide is effective
for severe asthma with noneosinoÂphilic phenotype. Eur Respir J 2008;31:1379-82. https://doi.org/10.1183/09031936.00014108
66. Szefler
SJ, Vogelber C, Bernstein JA et al. Tiotropium Is Efficacious in 6-to 17-Year-Olds with Asthma.
Independent of T2 Phenotype. J Allergy Clin Immunol Pract
2019;7:2286- 95.
https://doi.org/10.1016/j.jaip.2019.03.019
67. Wenzel S. Asthma phenotypes:
the evolution from clinical to molecular approaches. Nat Med 2012;18:716-25. https://doi.org/10.1038/nm.2678
68. Agache
IO. From phenotypes to endotypes to
asthma treatÂment. Curr Opin
Allergy Clin Immunol 2013;13: 249-56. https://doi.org/10.1097/ACI.0b013e32836093dd
69. Fitzpatrick AM, Moore WC.
Severe asthma phenotypes - How should they guide evaluation and treatment? J
Allergy Clin Immunol
Practice 2017;5:901-8.
https://doi.org/10.1016/j.jaip.2017.05.015
70. Farne
HA, Wilson A, Powell C, et al. Anti-IL5 therapies for asthma. Cochr Database Syst Rev 2017;9:CD010834. https://doi.org/10.1002/14651858.CD010834.pub3
71. Normansell
R, Walker S, Milan SJ, et al. Omalizumab for asthma
in adults and children (Review). Cochrane Database Syst
Rev 2014:CD003559. https://doi.org/10.1002/14651858.CD003559.pub4
72. SolÃĻr
M, Matz J, Townley R, et
al. The anti-IgE antibody omalizumab
reduces exacerbations and steroid requirement in allergic asthmatics. Eur Respir J. 2001;18:254-61. https://doi.org/10.1183/09031936.01.00092101
73. Busse
W, Corren J, Lanier BQ, et al. Omalizumab,
anti-IgE recombinant humanized monoclonal antibody,
for the treatÂment of severe allergic asthma. J Allergy Clin Immunol. 2001;108:184-90. https://doi.org/10.1067/mai.2001.117880
74.
Hanania NA, Alpan O, Hamilos DL, et al. Omalizumab in severe allergic
asthma inadequately controlled with standard therapy: A randomized trial. Ann Int Med 2011;154:573-82.
https://doi.org/10.7326/0003-4819-154-9-201105030-00002
75. Humbert
M, TaillÃĐ C, Mala L, Le Gros
V, Just J, Molimard M. Omalizumab
effectiveness in patients with severe alÂlergic asthma according to blood eosinophilic count: The STELLAIR study Eur
Respir J. 2018;51:1702523.
https://doi.org/10.1183/13993003.02523-2017
76. Casale
TB, Luskin AT, Busse W, et
al. Omalizumab effectiveness by biomarker status in
patients with asthma: Evidence from PROSPERO, a prospective rea-world
study. J Allergy Clin Immunol Pract. 2019;7:156-64. https://doi.org/10.1016/j.jaip.2018.04.043
77. Pavord
ID, Korn S, Howarth P, et
al. Mepolizumab for severe eosinophilic
asthma (DREAM): a multicentre, double-blind,
placebo-controlled trial. Lancet 2012;380:651-59.
https://doi.org/10.1016/S0140-6736(12)60988-X
78. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab
TreatÂment in Patients with Severe Eosinophilic
Asthma. N Engl J Med 2014;371:1198-207.
https://doi.org/10.1056/NEJMoa1403290
79. Bel
EH, Wenzel SE, Thompson PJ, et al. Oral GlucocorÂticoid-Sparing Effect of Mepolizumab in Eosinophilic
Asthma. N Engl J Med 2014;371:1189-97.
https://doi.org/10.1056/NEJMoa1403291
80. Khurana
S, Brusselle GG, Bel EH, et al. Long-term Safety and Clinical Benefit
of Mepolizumab in Patients With the Most Severe Eosinophilic Asthma: The COSMEX Study. Clinical Therapeutics;
2019;41:2041-56.
https://doi.org/10.1016/j.clinthera.2019.07.007
81. Harrison T, Canonica GW, Chupp G, et al.
Real-world mepolizumab in the prospective severe
asthma REALITI-A study: initial analysis. Eur Respir J 2020; 56: 2000151. https://doi.org/10.1183/13993003.00151-2020
82. FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab,
an anti-interleukin-5 receptor amonoclonal antibody,
as add-on treatment for patients with severe, uncontrolled, eoÂsinophilic
asthma (CALIMA): a randomized, double-blind, placebo-controlled phase 3 trial.
Lancet 2016;388:2128â41.
https://doi.org/10.1016/S0140-6736(16)31322-8
83. Bleecker
ER, FitzGerald JM, Chanez P, et al. Efficacy and
safety of benralizumab for patients with severe
asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting
à2-agonists (SIROCCO): a randomized, multicentre,
placebo-controlled phase 3 trial. Lancet Respir Med
2016;388:2115-27. https://doi.org/10.1016/S0140-6736(16)31324-1
84. FitzGerald JM, Bleecker ER, Menzies-Gow A, et
al. Predictors of enhanced response with benralizumab
for patients with severe asthma: pooled analysis of the SIROCCO and CALIMA
studies. Lancet Respir Med 2018;6:51-64.
https://doi.org/10.1016/S2213-2600(17)30344-2
85. Busse
WW, Bleecker ER, FitzGerald JM, et al. Long-term
safety and efficacy of benralizumab in patients with
severe, uncontrolled asthma: 1-year results from the BORA phase 3 extension trial. Lancet Respir Med 2019;7:46-59. https://doi.org/10.1016/S2213-2600(18)30406-5
86. Nair P, Wenzel S, Rabe KF et al. Oral Glucocorticoid-SparÂing Effect of Benralizumab in Severe Asthma. N Engl
J Med 2017;376:2448-58.
https://doi.org/10.1056/NEJMoa1703501
87. Korn
S, MDa, Bourdin A, Chupp G, et al. Integrated Safety and Efficacy Among Patients
Receiving Benralizumab for Up to 5 Years. J Allergy Clin Immunol Pract
2021;9:4381- 92.
https://doi.org/10.1016/j.jaip.2021.07.058
88. Wenzel S, Ford L, Pearlman D,
et al. Dupilumab in persisÂtent asthma with elevated
eosinophil levels. N Engl J Med 2013;368:2455-66. https://doi.org/10.1056/NEJMoa1304048
89. Wenzel S, Castro M, Corren J, et al. Dupilumab
efficacy and safety in adults with uncontrolled persistent asthma despite use
of medium-to-high-dose inhaled corticosteroids plus a long-acting B2 agonist: a
randomized double-blind placebo-controlled pivotal pase
2b dose-ranging trial. Lancet 2016;388:31-44.
https://doi.org/10.1016/S0140-6736(16)30307-5
90. Castro M, Corren
J, Pavord ID, et al. Dupilumab
Efficacy and Safety in Moderate-to-Severe Uncontrolled Asthma N Engl J Med. 2018;378:2486-96.
https://doi.org/10.1056/NEJMoa1804092
91. Rabe
KF, Nair P, Brusselle G, et al. Efficacy and Safety
of Dupilumab in Glucocorticoid-Dependent Severe
Asthma. N Engl J Med. 2018;378:2475-85.
https://doi.org/10.1056/NEJMoa1804093
92. Zayed
Y, Kheiri B, Banifadel M,
et al. Dupilumab safety and efficacy in uncontrolled
asthma: a systematic reÂview and meta-analysis of randomized clinical trials. J Asthma. 2018;1:1-10.
https://doi.org/10.1056/NEJMoa1804093
93. Bacharier
LB, Maspero JF, Katelaris
CH, Fiocchi AG, GaÂgnon R, de Mir I. Dupilumab in Children with Uncontrolled Moderate-to-Severe
Asthma. N Engl J Med 2021;385:2230-
40. https://doi.org/10.1056/NEJMoa2106567
94. Agache
I, Beltran J, Akdis C, Akdis
M, Canelo-Aybar C. Efficacy and safety of treatment
with biologicals (benraliÂzumab,
dupilumab, mepolizumab, omalizumab and resliÂzumab) for
severe eosinophilic asthma. A
systematic review for the EAACI Guidelines - recommendations on the use of biologicals in severe asthma. Allergy. 2020;75:1023-42. https://doi.org/10.1111/all.14221